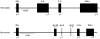Interleukin-26: an IL-10-related cytokine produced by Th17 cells - PubMed (original) (raw)
Review
Interleukin-26: an IL-10-related cytokine produced by Th17 cells
Raymond P Donnelly et al. Cytokine Growth Factor Rev. 2010 Oct.
Abstract
IL-26 is classified as a member of the IL-10 cytokine family because it has limited sequence homology to IL-10 and the IL-10-related cytokines. The human IL-26 gene, IL26, is located on chromosome 12q15 between the genes for two other important class-2 cytokines, IFNG (IFN-γ) and IL22 (IL-22). IL-26 is often co-expressed with IL-22 by activated T cells, especially Th17 cells. It signals through a heterodimeric receptor complex composed of the IL-20R1 and IL-10R2 chains. IL-26 receptors are primarily expressed on non-hematopoietic cell types, particularly epithelial cells. Signaling through IL-26 receptor complexes results in the activation of STAT1 and STAT3 with subsequent induction of IL-26-responsive genes. The biological functions of IL-26 have only begun to be defined.
Published by Elsevier Ltd.
Figures
Figure 1
A schematic comparison showing syntenic conservation of the IFN-γ, IL-26 and IL-22 loci in the human (Homo sapiens) and murine (Mus musculus) genomes. Information regarding the IFN-γ/IL-26/IL-22 locus was obtained from the NCBI map viewer for human chromosome-12 and murine chromosome-10. The closed boxes and arrowheads denote the positions and transcriptional orientations of the genes. The murine IL-26 gene fragments are shown as “open” boxes because they represent a bioinformatic prediction of an incomplete IL-26 gene. Abbreviations used: IFN-γ, interferon gamma; MDM, mouse double minute; IL, interleukin; ψ, pseudogene.
Figure 2
A comparison of the deduced amino acid sequences for the IL-26 gene in several mammalian and lower vertebrate species. Residues conserved across the sequences of multiple species are shaded. Dashes (−) indicate gaps that were introduced for optimal alignment. Multiple sequence alignment was carried out using Bio-edit software which uses Clustal W [version 1.8] and BLOSUM series was used for Protein weight matrix. The accession numbers for sequences used in this analysis are as follows: human, Homo sapiens, GenBank accession no. EAW97181; bovine, Bos taurus, GenBank accession no. XP_001250652; chimpanzee, Pan troglodytes, XP_001152032; rhesus monkey, Macaca mulatta, GenBank accession no. XP_001117154; zebrafish, Danio rerio, GenBank accession no. AAI63119; western clawed frog, Xenopus tropicalis, GenBank accession no. ABU54058.
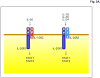
Figure 3
The IL-26 receptor complex. A. The IL-26 receptor complex is composed of two polypeptide chains: the ligand-binding chain, IL-20R1, and the accessory receptor chain, IL-10R2. IL-26 binds initially to IL-20R1, and then rapidly recruits the IL-10R2 chain to complete assembly of the active receptor complex. Ligand-induced heterodimerization of these receptor chains initiates a signal transduction cascade that results in the activation and nuclear translocation of STAT1 and STAT3. IL-20R1 can also dimerize with another class II cytokine receptor, IL-20R2, to generate receptors for IL-19, IL-20 and IL-24. B. IL-26 induces tyrosine phosphorylation of STAT1 and STAT3 in the human colorectal carcinoma cell line, COLO 205. COLO 205 cells were incubated with IL-22 or IL-26 (100 ng/mL) in the presence or absence of recombinant human IL-22 binding protein (IL-22BP) for 30 minutes at 37°C. At the end of this incubation period, whole cell lysates were prepared and analyzed by western blotting with antibodies specific for tyrosine-phosphorylated STAT1 or tyrosine-phosphorylated-STAT3 and total STAT1 and total STAT3.
Figure 3
The IL-26 receptor complex. A. The IL-26 receptor complex is composed of two polypeptide chains: the ligand-binding chain, IL-20R1, and the accessory receptor chain, IL-10R2. IL-26 binds initially to IL-20R1, and then rapidly recruits the IL-10R2 chain to complete assembly of the active receptor complex. Ligand-induced heterodimerization of these receptor chains initiates a signal transduction cascade that results in the activation and nuclear translocation of STAT1 and STAT3. IL-20R1 can also dimerize with another class II cytokine receptor, IL-20R2, to generate receptors for IL-19, IL-20 and IL-24. B. IL-26 induces tyrosine phosphorylation of STAT1 and STAT3 in the human colorectal carcinoma cell line, COLO 205. COLO 205 cells were incubated with IL-22 or IL-26 (100 ng/mL) in the presence or absence of recombinant human IL-22 binding protein (IL-22BP) for 30 minutes at 37°C. At the end of this incubation period, whole cell lysates were prepared and analyzed by western blotting with antibodies specific for tyrosine-phosphorylated STAT1 or tyrosine-phosphorylated-STAT3 and total STAT1 and total STAT3.
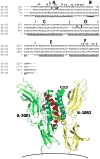
Figure 4
A structure-based sequence alignment of human IL-26 (A.) and a model of the human IL-26/IL-20R1/IL-10R2 ternary complex structure (B.). The sequence alignment was performed as described in the text. The six main helices (A–F) are denoted in Fig. 4A, and * and #s represent putative IL-20R1 and IL-10R2 binding residues, respectively. In the structure shown in Fig. 4B, helices A and B are colored red and purple, respectively. Cysteine residues are shown in yellow to denote residues that are conserved in the IL-19 sequence and green for residues that are not. The putative position of the cell membrane is shown at the bottom of the figure.
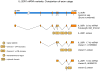
Figure 5
The IL-20R1 gene, IL20RA, encodes several RNA splice variants. Three variants of the IL-20R1 gene can be expressed in cells that express this receptor chain. The first is the full-length 3.6 kb transcript (GenBank accession no. NM_014432) that encodes the complete receptor polypeptide, including both fibronectin type-III (FnIII) domains of the extracellular region. The second is a 1.7 kb variant (GenBank accession no. AY358883) that lacks the membrane-distal FnIII domain, but retains the membrane-proximal FnIII domain, the trans-membrane domain, and the complete intracellular region. This variant arises as a result of deletion of most of exon 1 and all of exon 3. The third transcript is a 1.9 kb variant (GenBank accession no. AK098312) that lacks the entire extracellular region, but retains a small part of the trans-membrane domain and the complete intracellular domain. This variant arises as a result of the complete deletion of exons 1, 2 and 3. Although the full-length (3.6 kb) transcript encodes a functional IL-26-binding protein, the functions of the shorter 1.7 kb and 1.9 kb variants are unknown.
Similar articles
- Interleukin-26, a highly cationic T-cell cytokine targeting epithelial cells.
Braum O, Pirzer H, Fickenscher H. Braum O, et al. Antiinflamm Antiallergy Agents Med Chem. 2012;11(3):221-9. doi: 10.2174/1871523011202030221. Antiinflamm Antiallergy Agents Med Chem. 2012. PMID: 23106140 Review. - Cutting edge: IL-26 signals through a novel receptor complex composed of IL-20 receptor 1 and IL-10 receptor 2.
Sheikh F, Baurin VV, Lewis-Antes A, Shah NK, Smirnov SV, Anantha S, Dickensheets H, Dumoutier L, Renauld JC, Zdanov A, Donnelly RP, Kotenko SV. Sheikh F, et al. J Immunol. 2004 Feb 15;172(4):2006-10. doi: 10.4049/jimmunol.172.4.2006. J Immunol. 2004. PMID: 14764663 - The novel chicken interleukin 26 protein is overexpressed in T cells and induces proinflammatory cytokines.
Truong AD, Park B, Ban J, Hong YH. Truong AD, et al. Vet Res. 2016 Jun 16;47(1):65. doi: 10.1186/s13567-016-0342-0. Vet Res. 2016. PMID: 27312894 Free PMC article. - MicroRNAs: New regulators of IL-22.
Lu Z, Liu R, Huang E, Chu Y. Lu Z, et al. Cell Immunol. 2016 Jun-Jul;304-305:1-8. doi: 10.1016/j.cellimm.2016.05.003. Epub 2016 May 17. Cell Immunol. 2016. PMID: 27221197 Review. - Functional characterization of a nonmammalian IL-21: rainbow trout Oncorhynchus mykiss IL-21 upregulates the expression of the Th cell signature cytokines IFN-gamma, IL-10, and IL-22.
Wang T, Diaz-Rosales P, Costa MM, Campbell S, Snow M, Collet B, Martin SA, Secombes CJ. Wang T, et al. J Immunol. 2011 Jan 15;186(2):708-21. doi: 10.4049/jimmunol.1001203. Epub 2010 Dec 15. J Immunol. 2011. PMID: 21160047
Cited by
- Regulation of pulmonary graft-versus-host disease by IL-26+CD26+CD4 T lymphocytes.
Ohnuma K, Hatano R, Aune TM, Otsuka H, Iwata S, Dang NH, Yamada T, Morimoto C. Ohnuma K, et al. J Immunol. 2015 Apr 15;194(8):3697-712. doi: 10.4049/jimmunol.1402785. Epub 2015 Mar 18. J Immunol. 2015. PMID: 25786689 Free PMC article. - T helper 17-associated cytokines are produced during antigen-specific inflammation in the mammary gland.
Rainard P, Cunha P, Bougarn S, Fromageau A, Rossignol C, Gilbert FB, Berthon P. Rainard P, et al. PLoS One. 2013 May 16;8(5):e63471. doi: 10.1371/journal.pone.0063471. Print 2013. PLoS One. 2013. PMID: 23696826 Free PMC article. - Identification of interleukin-26 in the dromedary camel (Camelus dromedarius): Evidence of alternative splicing and isolation of novel splice variants.
Premraj A, Nautiyal B, Aleyas AG, Rasool TJ. Premraj A, et al. Mol Immunol. 2015 Oct;67(2 Pt B):357-68. doi: 10.1016/j.molimm.2015.06.022. Epub 2015 Jul 17. Mol Immunol. 2015. PMID: 26190308 Free PMC article. - Type 3 immunity: a perspective for the defense of the mammary gland against infections.
Rainard P, Cunha P, Martins RP, Gilbert FB, Germon P, Foucras G. Rainard P, et al. Vet Res. 2020 Oct 15;51(1):129. doi: 10.1186/s13567-020-00852-3. Vet Res. 2020. PMID: 33059767 Free PMC article. - IL-26 promotes the proliferation and survival of human gastric cancer cells by regulating the balance of STAT1 and STAT3 activation.
You W, Tang Q, Zhang C, Wu J, Gu C, Wu Z, Li X. You W, et al. PLoS One. 2013 May 21;8(5):e63588. doi: 10.1371/journal.pone.0063588. Print 2013. PLoS One. 2013. PMID: 23704922 Free PMC article. Retracted.
References
- Kotenko S. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 2002;13:223–240. - PubMed
- Renauld JC. Class II cytokine receptors and their ligands: key antiviral and inflammatory modulators. Nat Rev Immunol. 2003;3:667–676. - PubMed
- Fickenscher H, Pirzer H. Interleukin-26. Int Immunopharmacol. 2004;4:609–613. - PubMed
- Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immununol. 2007;8:950–957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI047300/AI/NIAID NIH HHS/United States
- AI47300/AI/NIAID NIH HHS/United States
- R21 AI081065/AI/NIAID NIH HHS/United States
- AI47300-08S/AI/NIAID NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous