Simultaneous disruption of two DNA polymerases, Polη and Polζ, in Avian DT40 cells unmasks the role of Polη in cellular response to various DNA lesions - PubMed (original) (raw)
Simultaneous disruption of two DNA polymerases, Polη and Polζ, in Avian DT40 cells unmasks the role of Polη in cellular response to various DNA lesions
Kouji Hirota et al. PLoS Genet. 2010.
Abstract
Replicative DNA polymerases are frequently stalled by DNA lesions. The resulting replication blockage is released by homologous recombination (HR) and translesion DNA synthesis (TLS). TLS employs specialized TLS polymerases to bypass DNA lesions. We provide striking in vivo evidence of the cooperation between DNA polymerase η, which is mutated in the variant form of the cancer predisposition disorder xeroderma pigmentosum (XP-V), and DNA polymerase ζ by generating POLη(-/-)/POLζ(-/-) cells from the chicken DT40 cell line. POLζ(-/-) cells are hypersensitive to a very wide range of DNA damaging agents, whereas XP-V cells exhibit moderate sensitivity to ultraviolet light (UV) only in the presence of caffeine treatment and exhibit no significant sensitivity to any other damaging agents. It is therefore widely believed that Polη plays a very specific role in cellular tolerance to UV-induced DNA damage. The evidence we present challenges this assumption. The phenotypic analysis of POLη(-/-)/POLζ(-/-) cells shows that, unexpectedly, the loss of Polη significantly rescued all mutant phenotypes of POLζ(-/-) cells and results in the restoration of the DNA damage tolerance by a backup pathway including HR. Taken together, Polη contributes to a much wide range of TLS events than had been predicted by the phenotype of XP-V cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
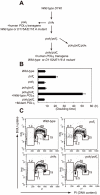
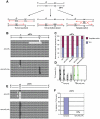
Figure 1. Proliferation of chicken DT40 polζ, polη, and polη/polζ cells.
(A) Schematic representation of the generation of the mutant cells used in this study. (B) The polη and polη/polζ mutants grew more rapidly than did the polζ cells. Cells were cultured for 3 days. The doubling time of each mutant is shown. Note that wild-type cells divide every 8 hours. “+_Wild-type POLη_” represents a reconstitution of polη/polζ cells with wild-type human polη. “_Mutant POLη_” carries D115A/E116A mutations, which inactivate the polymerase activity of human Polη. Error bars show the SD of the mean for three independent assays. (C) Representative asynchronous flow cytometric cell-cycle profiles of wild-type, polη polζ, and polη/polζ cells. Cells were pulse-labeled with BrdU for 10 minutes and stained with propidium iodide (PI) to measure DNA content. The large gate on the left of each panel indicates the sub-G1 apoptotic cell fraction. The lower left, arch, and lower right gates indicate cells in the G1, S and G2/M phase, respectively. Numbers show the percentage of cells falling in each gate.
Figure 2. Increased tolerance of polη/polζ cells to various genotoxic stresses, compared with polζ cells.
Colony survival of the indicated genotypes following the indicated genotoxic stresses. Data for the polη/polζ #1 clone are shown in the other figures. Error bars show the SD of the mean for three independent assays.
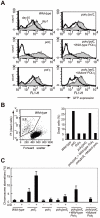
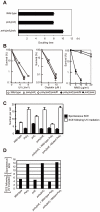
Figure 3. polζ cells, but not polη or polη/polζ cells, are hypersensitive to AID overexpression.
(A) Reduced AID transgene expression over time in polζ cells. The expression of AID was estimated by measuring the intensity of the GFP signal. Filled and open distributions represent GFP intensity at days 1 and 10 post-infection, respectively. (B) Accumulation of dead cells in the polζ mutant population 3 days after the AID virus infection. The dot plot shows cells stained with PI. The percentage of dead cells falling into the indicated gate is shown in the histogram. (C) Chromosomal aberrations are induced by AID overexpression in polζ mutants. At day 3 post-infection, chromosomal aberrations in mitotic cells of the indicated genotype were analyzed. Fifty metaphase spreads were measured. The number of aberrations per cell ± SE was calculated as x/N±√x/N, where N and x are the numbers of cells analyzed and total aberrations, respectively.
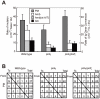
Figure 4. TLS–dependent point mutations in Ig V occur with comparable frequency in wild-type and polη/polζ cells.
(A) Rate of non-templated single-base substitutions (PM), ambiguous single base substitutions (Amb) (which are single base changes that have a potential donor sequence in the pseudogene array), and bona fide gene conversion tracts (GC) in the AID-virus-infected wild-type, polη, and polη/polζ DT40 cells shown in Figure 3. Cells were clonally expanded for two weeks. Three clones were analyzed in each data set. Note that the Amb mutation at A/T pairs represents short-tract gene conversion . (B) Nucleotide substitution preferences (as a percentage of the indicated number in the total mutations) deduced from the Vλ sequence analysis of the clones shown in (A). The preferences are shown for mutations categorized as non-templated base substitutions (PM), which are caused by TLS past abasic sites .
Figure 5. TLS past a T-T (6-4) UV photoproduct on episomal plasmid DNA.
(A) Schematic of the staggered arrangement of T–T (6–4) UV photoproducts in the construct pQTs. The dinucleotide GC is placed opposite each lesion, and there are 28 bp between the lesions, with the possible outcomes of DNA replication over the area. TLS may occur on either the top or the bottom strand, with the most common base insertion shown as AA. Alternatively, the nascent strand of the sister chromatid may be used as an alternative undamaged template; one possible mechanism for such a template switching illustrated. (B) Example sequences of replicated pQTs plasmids recovered from xpa/polζ and xpa/polη/polζ clones, aligned with a schematic drawing of pQTs. TLS on the top strand (inserting mostly AA in the reverse direction, top set), TLS on the bottom strand (middle set), and error-free bypass (bottom set) are shown. Proportions are not representative. (C) The proportion of TLS versus error-free bypass in pQTs sequences recovered from xpa/polζ and xpa/polη/polζ cells, shown as a percentage of the total. Data shown with asterisks are taken from Szüts et al. . A total of 23 and 29 sequences from xpa/polζ and xpa/polη/polζ cells, respectively, are shown as the sum of three independent experiments. (D) The pattern of nucleotide incorporation opposite the T-T (6-4) photoproduct in xpa/polζ and xpa/polη/polζ cells. Error-free pQTs sequences were excluded from analysis. The percentage of each nucleotide incorporated at each position is indicated by the size of the letter of the nucleotide in the column. del = deletion. The incorporation positions indicated are at the 3′ T and the 5′ T of the lesion, followed by the next two bases in the template, indicated by 1 and 2. (E) Example sequences of replicated pQTo plasmids recovered from xpa/polζ and xpa/polη/polζ clones, aligned with a schematic drawing of pQTo. (F) The frequency of replication associated with two or more nucleotide deletions in replicated copies of pQTo, which contains T–T (6–4) UV photoproducts in the opposing arrangement.
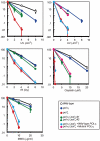
Figure 6. HR, but not Polκ, contributes to increased cellular tolerance to DNA damage in polη/polζ cells, compared with polζ cells.
(A) Growth of polη/polζ and polη/polζ/polκ clones. Cells were cultured for 3 days. The doubling time of each mutant is shown. Error bars show the SD of the mean for three independent assays. (B) Sensitivity profiles of indicated mutant cells to UV and cisplatin. Error bars show the SD of the mean for three independent assays. (C) Induction of SCE in polη, polζ, and polη/polζ cells by UV. Cells were labeled with BrdU during over cell-cycles with or without UV irradiation (0.25 J/m2) before labeling. Spontaneous and induced SCEs in the macrochromosomes of 50 metaphase cells were counted. Histograms show the frequency of cells with the indicated numbers of SCEs per cell. The mean number of SCEs per cell ± SE is shown. Statistical significance was further calculated by the non-parametric Mann-Whitney U test. (D) The number of UV-induced SCE was calculated by subtracting spontaneous SCE from SCE following UV irradiation.
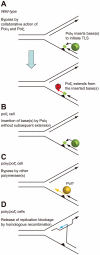
Figure 7. Model for the sequential action of Polη and Polζ in wild-type, polζ, and polη/polζ clones.
(A) The two step-model for TLS. In wild-type cells, Polη is efficiently recruited to a replication blockage at a DNA lesion (green circle) on a template strand and inserts 1∼2 base(s) (green box) to bypass the lesion (filled triangle). Polζ further extends from these inserted base(s) (red arrow) before replicative DNA polymerases can take over from Polζ. (B) In polζ cells, after Polη inserts nucleotides opposite to DNA lesions, no other polymerases can effectively extend DNA synthesis for subsequent DNA synthesis by replicative polymerases. (C) In polη/polζ cells, other TLS polymerases carry out TLS. (D) The replication blockage may be released by a template switch to the intact sister chromatid using the HR machinery.
Similar articles
- Stalled Polη at its cognate substrate initiates an alternative translesion synthesis pathway via interaction with REV1.
Ito W, Yokoi M, Sakayoshi N, Sakurai Y, Akagi J, Mitani H, Hanaoka F. Ito W, et al. Genes Cells. 2012 Feb;17(2):98-108. doi: 10.1111/j.1365-2443.2011.01576.x. Genes Cells. 2012. PMID: 22244149 - Interaction with DNA polymerase eta is required for nuclear accumulation of REV1 and suppression of spontaneous mutations in human cells.
Akagi J, Masutani C, Kataoka Y, Kan T, Ohashi E, Mori T, Ohmori H, Hanaoka F. Akagi J, et al. DNA Repair (Amst). 2009 May 1;8(5):585-99. doi: 10.1016/j.dnarep.2008.12.006. Epub 2009 Jan 21. DNA Repair (Amst). 2009. PMID: 19157994 - Genetic and physical interactions between Polη and Rev1 in response to UV-induced DNA damage in mammalian cells.
Bi T, Niu X, Qin C, Xiao W. Bi T, et al. Sci Rep. 2021 Nov 1;11(1):21364. doi: 10.1038/s41598-021-00878-3. Sci Rep. 2021. PMID: 34725419 Free PMC article. - Xeroderma pigmentosum variant and error-prone DNA polymerases.
Kannouche P, Stary A. Kannouche P, et al. Biochimie. 2003 Nov;85(11):1123-32. doi: 10.1016/j.biochi.2003.10.009. Biochimie. 2003. PMID: 14726018 Review. - Multiple two-polymerase mechanisms in mammalian translesion DNA synthesis.
Livneh Z, Ziv O, Shachar S. Livneh Z, et al. Cell Cycle. 2010 Feb 15;9(4):729-35. doi: 10.4161/cc.9.4.10727. Epub 2010 Feb 23. Cell Cycle. 2010. PMID: 20139724 Review.
Cited by
- Cell-based high-throughput screens for the discovery of chemotherapeutic agents.
Fox JT, Myung K. Fox JT, et al. Oncotarget. 2012 May;3(5):581-5. doi: 10.18632/oncotarget.513. Oncotarget. 2012. PMID: 22653910 Free PMC article. - PrimPol bypasses UV photoproducts during eukaryotic chromosomal DNA replication.
Bianchi J, Rudd SG, Jozwiakowski SK, Bailey LJ, Soura V, Taylor E, Stevanovic I, Green AJ, Stracker TH, Lindsay HD, Doherty AJ. Bianchi J, et al. Mol Cell. 2013 Nov 21;52(4):566-73. doi: 10.1016/j.molcel.2013.10.035. Mol Cell. 2013. PMID: 24267451 Free PMC article. - DNA repair mechanisms and the bypass of DNA damage in Saccharomyces cerevisiae.
Boiteux S, Jinks-Robertson S. Boiteux S, et al. Genetics. 2013 Apr;193(4):1025-64. doi: 10.1534/genetics.112.145219. Genetics. 2013. PMID: 23547164 Free PMC article. Review. - PDIP38/PolDIP2 controls the DNA damage tolerance pathways by increasing the relative usage of translesion DNA synthesis over template switching.
Tsuda M, Ogawa S, Ooka M, Kobayashi K, Hirota K, Wakasugi M, Matsunaga T, Sakuma T, Yamamoto T, Chikuma S, Sasanuma H, Debatisse M, Doherty AJ, Fuchs RP, Takeda S. Tsuda M, et al. PLoS One. 2019 Mar 6;14(3):e0213383. doi: 10.1371/journal.pone.0213383. eCollection 2019. PLoS One. 2019. PMID: 30840704 Free PMC article. - Eukaryotic DNA Polymerases in Homologous Recombination.
McVey M, Khodaverdian VY, Meyer D, Cerqueira PG, Heyer WD. McVey M, et al. Annu Rev Genet. 2016 Nov 23;50:393-421. doi: 10.1146/annurev-genet-120215-035243. Annu Rev Genet. 2016. PMID: 27893960 Free PMC article. Review.
References
- Friedberg EC, Lehmann AR, Fuchs RP. Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol Cell. 2005;18:499–505. - PubMed
- Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. - PubMed
- Johnson RE, Kondratick CM, Prakash S, Prakash L. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science. 1999;285:263–265. - PubMed
- Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, et al. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature. 1999;399:700–704. - PubMed
- Lehmann AR. Replication of damaged DNA in mammalian cells: new solutions to an old problem. Mutat Res. 2002;509:23–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources