Recognition of nuclear targeting signals by Karyopherin-β proteins - PubMed (original) (raw)
Review
Recognition of nuclear targeting signals by Karyopherin-β proteins
Darui Xu et al. Curr Opin Struct Biol. 2010 Dec.
Abstract
The Karyopherin-β family of nuclear transport factors mediates the majority of nucleocytoplasmic transport. Although each of the 19 Karyopherin-βs transports unique sets of cargos, only three classes of nuclear localization and export signals, or NLSs and NESs, have been characterized. The short basic classical-NLS was first discovered in the 1980s and their karyopherin-bound structures were first reported more than 10 years ago. More recently, structural and biophysical studies of Karyopherin-β2-cargo complexes led to definition of the complex and diverse PY-NLS. Structural knowledge of the leucine-rich NES is finally available more than 10 years after the discovery of its recognition by the exportin CRM1. We review recent findings relating to how these three classes of nuclear targeting signals are recognized by their Karyopherin-β nuclear transport factors.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
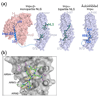
Figure 1
Classical-NLS recognition by Impα/β. (a) Impβ (pink) binds to the IBB domain of Impα (blue ribbon); PDB ID 1QGK. Monopartite (1EJL) or bipartite (1EJY) classical-NLSs (green) bind to the Impα ARM domain (surface representation in blue). Full length Impα is autoinhibited. An NLS-like N-terminal segment (blue sticks) occupies the major NLS binding site on the ARM domain (blue surface) and prohibits binding of exogeneous NLS; PDB ID 1IAL. A dashed blue line represents the unstructured polypeptide chain that connects the Impα ARM domain to its IBB domain. (b) Interactions between the SV40 T antigen NLS (green) and the major binding site of Impα (grey; PDB ID 1BK6). Conserved tryptophan-asparagine pairs and acidic residues in Impα are colored yellow and magenta, respectively.
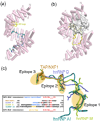
Figure 2
The Kapβ2 nuclear import pathway. (a) Crystal structure of Kapβ2 (pink) bound to the PY-NLS of hnRNP A1 (green; also known as the M9 sequence); PDB ID 2H4M. The internal loop in HEAT repeat 8 is mostly disordered and colored yellow. (b) Crystal structure of Kapβ2 (pink) bound to Ran•GppNHp (grey surface); PDB ID 1QBK. When Ran is bound, the H8 loop (yellow) is mostly ordered and binds to the PY-NLS binding site. (c) Structures of PY-NLSs from hnRNPs A1 (green; 2H4M), M (yellow-green; 2OT8), D (blue; 2Z5N) and mRNA export factor TAP/NXF1 (orange; 2Z5K) upon superposition of Kapβ2s. All four peptides spatially converge at epitopes 1, 2 and 3, which are connected by structurally variable linkers. PY-NLS sequences are shown in the box, with residues in epitope 1 shown in yellow (hydrophobic motif) and blue (basic motif) and conserved residues in epitopes 2 and 3 shown in red.
Figure 3
Structures of CRM1 complexes. The structure of CRM1 (pink, surface representation) bound with cargo SNUPN (light blue ribbon) with (a, PDB ID 3GJX) and without (b, PDB ID 3GB8) RanGTP (grey). (c) The structure of CRM1 (pink) bound with RanGTP (grey) and RanBP1 (green; PDB ID 3M1I). (d) The leucine-rich NES of SNUPN (light blue ribbon) bound to the hydrophobic groove of CRM1 (pink). The helices of HEAT repeats 11 and 12 are shown as ribbons and sidechains that line the hydrophobic groove are shown. (e) Rearrangement of the hydrophobic groove in the CRM1-RanGTP-RanBP1 complex. Helices of HEAT repeats 11 and 12 are shown as in (d). Helix movement resulted in narrower or ‘closed’ hydrophobic groove, which is incompatible with NES binding.
Similar articles
- Nuclear localization signals for four distinct karyopherin-β nuclear import systems.
Soniat M, Chook YM. Soniat M, et al. Biochem J. 2015 Jun 15;468(3):353-62. doi: 10.1042/BJ20150368. Biochem J. 2015. PMID: 26173234 Review. - Rules for nuclear localization sequence recognition by karyopherin beta 2.
Lee BJ, Cansizoglu AE, Süel KE, Louis TH, Zhang Z, Chook YM. Lee BJ, et al. Cell. 2006 Aug 11;126(3):543-58. doi: 10.1016/j.cell.2006.05.049. Cell. 2006. PMID: 16901787 Free PMC article. - Crystal structure of human Karyopherin β2 bound to the PY-NLS of Saccharomyces cerevisiae Nab2.
Soniat M, Sampathkumar P, Collett G, Gizzi AS, Banu RN, Bhosle RC, Chamala S, Chowdhury S, Fiser A, Glenn AS, Hammonds J, Hillerich B, Khafizov K, Love JD, Matikainen B, Seidel RD, Toro R, Rajesh Kumar P, Bonanno JB, Chook YM, Almo SC. Soniat M, et al. J Struct Funct Genomics. 2013 Jun;14(2):31-5. doi: 10.1007/s10969-013-9150-1. Epub 2013 Mar 28. J Struct Funct Genomics. 2013. PMID: 23535894 Free PMC article. - A PY-NLS nuclear targeting signal is required for nuclear localization and function of the Saccharomyces cerevisiae mRNA-binding protein Hrp1.
Lange A, Mills RE, Devine SE, Corbett AH. Lange A, et al. J Biol Chem. 2008 May 9;283(19):12926-34. doi: 10.1074/jbc.M800898200. Epub 2008 Mar 14. J Biol Chem. 2008. PMID: 18343812 Free PMC article. - Nuclear import by karyopherin-βs: recognition and inhibition.
Chook YM, Süel KE. Chook YM, et al. Biochim Biophys Acta. 2011 Sep;1813(9):1593-606. doi: 10.1016/j.bbamcr.2010.10.014. Epub 2010 Oct 26. Biochim Biophys Acta. 2011. PMID: 21029754 Free PMC article. Review.
Cited by
- Recognition Elements in the Histone H3 and H4 Tails for Seven Different Importins.
Soniat M, Cağatay T, Chook YM. Soniat M, et al. J Biol Chem. 2016 Sep 30;291(40):21171-21183. doi: 10.1074/jbc.M116.730218. Epub 2016 Aug 15. J Biol Chem. 2016. PMID: 27528606 Free PMC article. - The Nuclear Pore Complex Is a Key Target of Viral Proteases to Promote Viral Replication.
De Jesús-González LA, Palacios-Rápalo S, Reyes-Ruiz JM, Osuna-Ramos JF, Cordero-Rivera CD, Farfan-Morales CN, Gutiérrez-Escolano AL, Del Ángel RM. De Jesús-González LA, et al. Viruses. 2021 Apr 19;13(4):706. doi: 10.3390/v13040706. Viruses. 2021. PMID: 33921849 Free PMC article. Review. - Autographa californica Multiple Nucleopolyhedrovirus Ac34 Protein Retains Cellular Actin-Related Protein 2/3 Complex in the Nucleus by Subversion of CRM1-Dependent Nuclear Export.
Mu J, Zhang Y, Hu Y, Hu X, Zhou Y, Zhao H, Pei R, Wu C, Chen J, Zhao H, Yang K, Oers MM, Chen X, Wang Y. Mu J, et al. PLoS Pathog. 2016 Nov 1;12(11):e1005994. doi: 10.1371/journal.ppat.1005994. eCollection 2016 Nov. PLoS Pathog. 2016. PMID: 27802336 Free PMC article. - A non-canonical mechanism for Crm1-export cargo complex assembly.
Fischer U, Schäuble N, Schütz S, Altvater M, Chang Y, Faza MB, Panse VG. Fischer U, et al. Elife. 2015 Apr 21;4:e05745. doi: 10.7554/eLife.05745. Elife. 2015. PMID: 25895666 Free PMC article. - Cytoplasmic retention and degradation of a mitotic inducer enable plant infection by a pathogenic fungus.
Bardetti P, Castanheira SM, Valerius O, Braus GH, Pérez-Martín J. Bardetti P, et al. Elife. 2019 Oct 17;8:e48943. doi: 10.7554/eLife.48943. Elife. 2019. PMID: 31621584 Free PMC article.
References
- Tran EJ, Bolger TA, Wente SR. SnapShot: nuclear transport. Cell. 2007;131:420. - PubMed
- Chook YM, Blobel G. Karyopherins and nuclear import. Curr Opin Struct Biol. 2001;11:703–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-GM069909/GM/NIGMS NIH HHS/United States
- T32 GM007062/GM/NIGMS NIH HHS/United States
- R01 GM069909-08/GM/NIGMS NIH HHS/United States
- GM007062/GM/NIGMS NIH HHS/United States
- R01 GM069909/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources