Phosphatidylinositol-4,5-bisphosphate promotes budding yeast septin filament assembly and organization - PubMed (original) (raw)
Phosphatidylinositol-4,5-bisphosphate promotes budding yeast septin filament assembly and organization
Aurélie Bertin et al. J Mol Biol. 2010.
Abstract
Septins are a conserved family of GTP-binding proteins that assemble into symmetric linear heterooligomeric complexes, which in turn are able to polymerize into apolar filaments and higher-order structures. In budding yeast (Saccharomyces cerevisiae) and other eukaryotes, proper septin organization is essential for processes that involve membrane remodeling, such as the execution of cytokinesis. In yeast, four septin subunits form a Cdc11-Cdc12-Cdc3-Cdc10-Cdc10-Cdc3-Cdc12-Cdc11 heterooctameric rod that polymerizes into filaments thought to form a collar around the bud neck in close contact with the inner surface of the plasma membrane. To explore septin-membrane interactions, we examined the effect of lipid monolayers on septin organization at the ultrastructural level using electron microscopy. Using this methodology, we have acquired new insights into the potential effect of septin-membrane interactions on filament assembly and, more specifically, on the role of phosphoinositides. Our studies demonstrate that budding yeast septins interact specifically with phosphatidylinositol-4,5-bisphosphate (PIP2) and indicate that the N terminus of Cdc10 makes a major contribution to the interaction of septin filaments with PIP2. Furthermore, we found that the presence of PIP2 promotes filament polymerization and organization on monolayers, even under conditions that prevent filament formation in solution or for mutants that prevent filament formation in solution. In the extreme case of septin complexes lacking the normally terminal subunit Cdc11 or the normally central Cdc10 doublet, the combination of the PIP2-containing monolayer and nucleotide permitted filament formation in vitro via atypical Cdc12-Cdc12 and Cdc3-Cdc3 interactions, respectively.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
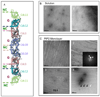
Figure 1. Effect of PI(4,5)P2-DOPC lipid monolayers on the assembly and organization of septin filaments. Scales bars: 100 nm
A. Schematic representation of the arrangement of the septin monomers into the octameric complex. The alternation of G (GTP or GDP binding) and NC interfaces are visualized. B. Wild type septins in vitro. Panel 1.Octameric budding yeast septin complexes in high salt (Tris-HCl pH 8, 50 mM, NaCl 300 mM) and negatively stained. Panel 2. Paired filaments, “Railroad tracks”, of septins in low salt conditions (Tris-HCl pH 8, 50 mM). C. Wild type septins on lipid monolayers. Panel 1 Filaments of septins at an initial concentration in solution of 0.01 mg.mL−1, with a weight PI(4,5)P2 concentration of 20 %. Septin filaments are tightly paired and start forming sheets of filaments. Panel 2. Filaments of septins organized into a flat sheet of filaments, at an initial concentration in solution of 0.03 mg.mL−1, with a weight PI(4,5)P2 concentration of 20 %. The Fourier transform of the image shows two repeats: 376 and 44.3 Å, corresponding to a septin octameric and monomeric repeat, respectively. Panel 3 Filaments of septin observed after an overnight incubation, an initial septin concentration of 0.005 mg.mL−1 and a PI(4,5)P2 concentration of 20 %. Tight paired filaments are connected by short rods, which length is 36.7 ± 7 nm and which are 36.5 ± 5 nm apart. Short octameric septin rods connect sets of parallel paired filaments orthogonally. Panel 4. Filaments of septin observed after an overnight incubation, an initial septin concentration of 0.02 mg.mL−1 and a PI(4,5)P2 concentration of 20 %. Arrows point connections between the ends of paired filaments to another perpendicular paired filament and are about 70 nm apart, which is the size of two septin octamers.
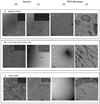
Figure 2. PI(4,5)P2 lipid monolayers facilitate filament formation
Scale bars: 100 nm long. 2.A. Filaments and sheets of filaments of septins in high salt conditions (Tris-HCl, pH8, 50 mM, NaCl 300 mM) with a starting concentration of 0.02 mg.mL−1 on DOPC lipid monolayers doped with 20 % PI(4,5)P2. 2.B. Δα0Cdc11 septin complexes in low salt (Tris-HCl 50 mM, with a starting concentration of 0.005 mg.mL−1 can form filaments on PIP2 (20% in DOPC) lipid monolayers. A periodic and octameric decoration can be visualized (white arrows). Short octameric rods are able to connect tight paired filaments (black arrows).
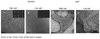
Figure 3. Assembly of ΔCTE septins
Scale bars: 100 nm. In solution (A, panels 1–2, B, panels 1–2, C, panels 1–2) and on lipid monolayers (A, panel 3;B, panel 3;C, panel 3) for Cdc11 (Δ 306–415)-Cdc3-Cdc12-Cdc10 (A), MBP-Cdc12 (Δ 318–407) -Cdc3 - Cdc10- Cdc11 (B) and (Cdc12 (Δ 318–407) -Cdc3 (Δ 419–520)- Cdc10- Cdc11 (C). For all lipid monolayers assay, DOPC lipid monolayers contained 20% PIP2 and proteins were incubated overnight with lipids. A, panel 1. Cdc11 (Δ 306–415)-Cdc3-Cdc12-Cdc10 septins form octameric rods in high salt conditions and paired filaments in low salt (A, panel 2). A, panel 3. On lipid monolayers, septin complexes with a missing coiled coil at the C-terminus of Cdc11 form filaments. The octameric decoration of the filaments is not visible and sheets of tight filaments do not form. B, panel 1. MBP-Cdc12 (Δ 318–407) -Cdc3- Cdc10- Cdc11 septin form stable octameric rods,in high salt conditions. Some of the class averages are shown on the inset, where Cdc12 displays an extra density that corresponds to the MBP tag. B, panel2. Septins without the Cdc12 C-terminal extension, in low salt solution do not assemble into long paired filaments. Instead they form short single filaments (of the size of 3–4 multiple of octamers), which can occasionally pair. B, panel3. Septins without the Cdc12 C-terminal extension, in low salt solution on lipid monolayers. Septins can form filaments and lattices of interconnected and perpendicular ones. C, panel 1. Septins without both of Cdc12 and Cdc3 C-terminal extensions, in high salt solution still form octameric rods. From 2D classification and averaging from a dataset of 1,400 particles, 50 % of octamers, 31.5 % of heptamers and 18.5% of shorter hexamers are obtained. Class averages are shown on the inset. C, panel 2. Septins without both of Cdc12 and Cdc3 C-terminal extensions, in low salt solution do not assemble into paired filaments. C, panel 3. Septins without both of Cdc12 and Cdc3 C-terminal extensions, in low salt solution on a PIP2-DOPC lipid monolayers assemble into tight paired filaments and extended sheets.
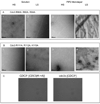
Figure 4. Effect of nucleotides and PI(4,5)P2 on the assembly of the Cdc11-less and Cdc10-less septin
Scale bars:100 nm. A. Cdc11-less septins organized on lipid monolayers with a PIP2 concentration of 20 % and a septin concentration 0f 0.03 mg.mL−1, in low salt conditions. A, panel 1. No nucleotide is added. A, panel 2. GTP 0.5mM is present. In A, panel 3 and B, panel 4, GDP 0.5 mM and GTP gamma-S are respectively added at a concentration of 0.5 mM. B. Cdc10-less, Cdc11-Cdc12-Cdc3(G261V) septin complexes organized on lipid monolayers with a PI(4,5)P2 concentration of 20% and a septin concentration of 0.02 mg.mL−1, in low salt conditions with (B, panel 2) or without (B, panel 1) additional GTP 0.5 mM.
Figure 5. Periodic decoration of septin bundles by PI(4,5)P2 micelles
Scale bars in A and B: 100 nm, scale bar in C: 20 nm A. Micelles of PIP2 in low salt conditions. B. Decoration of septin bundles by PIP2 micelles with an octameric repeat. C. The orange arrows point at the bundle tips while the black arrows point at the lipid micelles.
Figure 6. mutations in the alpha 0 helix of Cdc10
Scale bars: 100 nm. Cdc10 in complex with Cdc3, Cdc11 and Cdc12 was mutated in its alpha0 helix. The alpha0 helix was either deleted from amino acid 12 to 28 (A) or point mutations were designed. Basic residues were mutated to alanine (H24A, R25A, K28A, K29A Cdc10) (B). Isoleucine 28 was mutated to glutamic acid (C)). Those complexes were analyzed either in solution (A, panels 1–2; B, panels 1–2; C, panels 1–2) or on lipid monolayers of 20 % PIP2 in DOPC (A, panels 3–4; B, panels 3–4, C, panels 3–4). Representative 2-D class averages are shown on the right hand inset of each image when septin complexes are observed in solution. In solution and high salt, tetrameric complexes are mainly observed. The point mutants display a small percentage of longer rods as compared with the deletion of the whole alpha0 helix (A, panel 1; B, panel 1; C, panel 1). In solution and low salt conditions, short paired filaments can rarely be seen for the point mutants (B, panel 2 and C, panel 2, black arrows). Class averages show a kink in the middle of some of the longer rods (white arrows). On 20% PIP2 lipid monolayers with a starting septin concentration of 0.02 mg.mL−1, filaments can be recovered for the point mutants, in low salt (B, panel 4; C, panel 4). However those filaments were more rarely observed for the I22E mutation (C, panel 4). The Cdc10(Δα0)-Cdc11-Cdc12-Cdc3 does not polymerize on lipid monnolayers (A, panel 4).
Figure 7. Mutation in the alpha0 helix of Cdc11
Scale bars: 100 nm. The Cdc11 (R12A K13A R14A K15A K16A) Cdc11 mutant was analyzed in solution (A–B) and on lipid monolayers (C–D) in high salt (A and C) and low salt conditions (B and D). In solution, octameric rods are mainly observed in both high salt and low salt. Typical class averages are shown on the insets. On lipid monolayers tight paired filaments can be seen.
Figure 8. Cdc3 mutants
Scale bars: 100 nm. Cdc3 (R90A, R93A, R94A)–Cdc10–Cdc11-Cdc12 (A) and Cdc3 (R111A, R112A, K115A)–Cdc10–Cdc11-Cdc12 (B) were analyzed both in solution (A, panels 1–2; B, panels 1–2) and on DOPC lipid monolayers doped with PIP2 20 % (A, panels 3–4; B, panels 3–4). In solution, both of the mutants assemble into octameric rods in high salt (A, panel 1 and B, panel 1) and into paired filaments (A, panel 2 and B, panel 2) in low salt conditions. Samples were incubated on lipid monolayers, overnight, with a starting septin concentration of 0.02 mg.mL−1 in either low salt (A, panel 3; B, panel 3) or high salt (A, panel 4; B, panel 4) conditions. The Cdc3 (R90A, R93A, R94A)–Cdc10–Cdc11-Cdc12 mutant can form filaments and even sheets of filaments on lipid monolayers (A, panels 3–4). Cdc3 (R111A, R112A, K115A)–Cdc10–Cdc11-Cdc12 assembles into filaments on lipid monolayers (B, panels 3–4) at 0.02 mg.mL−1 as well. C. Budding yeast cells grown at 37 °C, wild type (C, panel 2) or mutants Cdc3(R90A, R93A, R94A R111A, R112A, K115A) (C, panel 1). No difference is discernible.
Similar articles
- Septin filament formation is essential in budding yeast.
McMurray MA, Bertin A, Garcia G 3rd, Lam L, Nogales E, Thorner J. McMurray MA, et al. Dev Cell. 2011 Apr 19;20(4):540-9. doi: 10.1016/j.devcel.2011.02.004. Dev Cell. 2011. PMID: 21497764 Free PMC article. - Guanidine hydrochloride reactivates an ancient septin hetero-oligomer assembly pathway in budding yeast.
Johnson CR, Steingesser MG, Weems AD, Khan A, Gladfelter A, Bertin A, McMurray MA. Johnson CR, et al. Elife. 2020 Jan 28;9:e54355. doi: 10.7554/eLife.54355. Elife. 2020. PMID: 31990274 Free PMC article. - Protein-protein interactions governing septin heteropentamer assembly and septin filament organization in Saccharomyces cerevisiae.
Versele M, Gullbrand B, Shulewitz MJ, Cid VJ, Bahmanyar S, Chen RE, Barth P, Alber T, Thorner J. Versele M, et al. Mol Biol Cell. 2004 Oct;15(10):4568-83. doi: 10.1091/mbc.e04-04-0330. Epub 2004 Jul 28. Mol Biol Cell. 2004. PMID: 15282341 Free PMC article. - Reuse, replace, recycle. Specificity in subunit inheritance and assembly of higher-order septin structures during mitotic and meiotic division in budding yeast.
McMurray MA, Thorner J. McMurray MA, et al. Cell Cycle. 2009 Jan 15;8(2):195-203. doi: 10.4161/cc.8.2.7381. Cell Cycle. 2009. PMID: 19164941 Free PMC article. Review. - Some assembly required: yeast septins provide the instruction manual.
Versele M, Thorner J. Versele M, et al. Trends Cell Biol. 2005 Aug;15(8):414-24. doi: 10.1016/j.tcb.2005.06.007. Trends Cell Biol. 2005. PMID: 16009555 Free PMC article. Review.
Cited by
- The septin cytoskeleton facilitates membrane retraction during motility and blebbing.
Gilden JK, Peck S, Chen YC, Krummel MF. Gilden JK, et al. J Cell Biol. 2012 Jan 9;196(1):103-14. doi: 10.1083/jcb.201105127. J Cell Biol. 2012. PMID: 22232702 Free PMC article. - The Roles of Septins in Regulating Fission Yeast Cytokinesis.
Zheng S, Zheng B, Fu C. Zheng S, et al. J Fungi (Basel). 2024 Jan 30;10(2):115. doi: 10.3390/jof10020115. J Fungi (Basel). 2024. PMID: 38392788 Free PMC article. Review. - Three-dimensional ultrastructure of the septin filament network in Saccharomyces cerevisiae.
Bertin A, McMurray MA, Pierson J, Thai L, McDonald KL, Zehr EA, García G 3rd, Peters P, Thorner J, Nogales E. Bertin A, et al. Mol Biol Cell. 2012 Feb;23(3):423-32. doi: 10.1091/mbc.E11-10-0850. Epub 2011 Dec 7. Mol Biol Cell. 2012. PMID: 22160597 Free PMC article. - Central Role of the Actomyosin Ring in Coordinating Cytokinesis Steps in Budding Yeast.
Foltman M, Sanchez-Diaz A. Foltman M, et al. J Fungi (Basel). 2024 Sep 21;10(9):662. doi: 10.3390/jof10090662. J Fungi (Basel). 2024. PMID: 39330421 Free PMC article. Review. - Cytokinetic contractile ring structural progression in an early embryo: positioning of scaffolding proteins, recruitment of α-actinin, and effects of myosin II inhibition.
Henson JH, Reyes G, Lo NT, Herrera K, McKim QW, Herzon HY, Galvez-Ceron M, Hershey AE, Kim RS, Shuster CB. Henson JH, et al. Front Cell Dev Biol. 2024 Sep 27;12:1483345. doi: 10.3389/fcell.2024.1483345. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39398481 Free PMC article.
References
- Hartwell LH. Genetic control of the cell division cycle in yeast. II. Genes controlling DNA replication and its initiation. J Mol Biol. 1971;59:183–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM021841/GM/NIGMS NIH HHS/United States
- R01 GM21841/GM/NIGMS NIH HHS/United States
- K99 GM86603/GM/NIGMS NIH HHS/United States
- K99 GM086603/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R00 GM086603/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases