Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons - PubMed (original) (raw)
Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons
Vladimir Camarena et al. Cell Host Microbe. 2010.
Erratum in
- Cell Host Microbe. 2010 Dec 16;8(6):551
Abstract
Herpes simplex virus-1 (HSV-1) establishes life-long latency in peripheral neurons where productive replication is suppressed. While periodic reactivation results in virus production, the molecular basis of neuronal latency remains incompletely understood. Using a primary neuronal culture system of HSV-1 latency and reactivation, we show that continuous signaling through the phosphatidylinositol 3-kinase (PI3-K) pathway triggered by nerve growth factor (NGF)-binding to the TrkA receptor tyrosine kinase (RTK) is instrumental in maintaining latent HSV-1. The PI3-K p110α catalytic subunit, but not the β or δ isoforms, is specifically required to activate 3-phosphoinositide-dependent protein kinase-1 (PDK1) and sustain latency. Disrupting this pathway leads to virus reactivation. EGF and GDNF, two other growth factors capable of activating PI3-K and PDK1 but that differ from NGF in their ability to persistently activate Akt, do not fully support HSV-1 latency. Thus, the nature of RTK signaling is a critical host parameter that regulates the HSV-1 latent-lytic switch.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
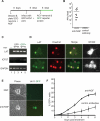
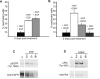
Fig 1. Establishment of a non-replicating HSV-1 infection in cultured SCG neurons
(A) Protocol for establishment of HSV-1 infections in SCG cultures. Dissociated SCG were seeded in 96-well plates in media supplemented with 50 ng/ml NGF. After 6 d, cells were treated with 50 μM acyclovir (ACV), and 1 d later infected with HSV-1 EGFP-Us11 (MOI = 1 based upon titer on Vero cells). Infected cultures were maintained with ACV for an additional week to allow the virus to establish a non-replicating infection. (B) Plaque assays were performed after 6 d of treatment with blocking antibodies against NGF or BDNF (control). Twenty individual wells were assayed for each condition. Bar show the average number of plaque forming units (PFU) per well. (C) Detection of HSV-1 transcripts by RT-PCR. After establishing a non-replicating HSV-1 infection in the presence of NGF and ACV, the ACV was removed and GFP fluorescence monitored over a 7 d period. RNA was collected from two GFP-negative samples (lanes 2 and 3) representing latently-infected cultures and two GFP-positive samples (lanes 4 and 5) representing the small number of cultures that spontaneously reactivated in the absence of any treatment. Semi-quantitative RT-PCR was used to determine the expression of LAT, ICP27 and cellular GAPDH RNA transcripts. Lane 1, uninfected SCG culture. (D) Accumulation of LAT in nuclei of latently-infected neurons. HSV-1-infections were established as described in (A). After ACV removal, neurons were fixed and probed with a combination of fluorescent in situ hybridization (FISH) and indirect-immunofluorescence. Viral LAT RNA transcripts were detected by RNA FISH [green] and nuclear DNA was visualized using Hoechst stain [red]. The merge of the RNA FISH and Hoechst signals highlights the latently-infected neurons [yellow]. Neurofilament protein NF200, a neuronal cytoplasmic marker, was detected by indirect immunofluorescence [white]. Bar = 20 μm. (E) Detection of reactivating virus by fluorescent microscopy. Phase contrast and fluorescent images of representative clusters of latently-infected neurons. Reactivation was induced by NGF-deprivation, resulting in the expression of the HSV-1 EGFP-Us11 fusion protein as a true late (γ2) gene product. The image was captured on day 3 following NGF-depletion. Bar = 20 μm. (F) Reactivation assay showing the percentage of wells containing GFP-positive cells detected by fluorescent microscopy of living neurons over a 6 d period after the removal of ACV and NGF. NGF was depleted using anti-NGF blocking antibodies and compared with anti-BDNF (control) antibodies.
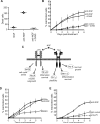
Figure 2. Inhibition of TrkA induces HSV-1 reactivation
(A) Pan-caspase inhibitor Z-VAD-fmk does not prevent the reactivation after NGF withdrawal. GFP-HSV-1 infected SCG neuron cultures were treated with anti-NGF or control antibodies in the presence or absence of 20 μM Z-VAD-fmk. Plot shows the number of GFP+ wells, indicative of reactivated virus, scored over a 6 d period. (B) Z-VAD-fmk was effective in blocking apoptosis. Cultured neurons were treated with anti-NGF antibody in the presence or absence of Z-VAD-fmk (20 μM). After 48 h, neurons were stained with Hoechst 33342 dye and scored for nuclear fragmentation indicative of apoptosis. Bar indicates mean (n = 200). (C) Schematic showing signaling pathways activated by NGF. SCG neurons express two NGF receptors, p75 or TrkA and activate multiple pathways that give rise to different biological effects (Chao, 2003). (D) Reactivation assay comparing treatments with the Trk inhibitor K252a (200 nM in DMSO), anti-NGF antibody (positive control) or DMSO alone. See also Figure S1. (E) Reactivation assay comparing anti-p75 (9651) blocking antibody with anti-NGF and IgG control antibodies.
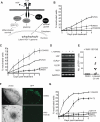
Figure 3. PI3-K is required in latency
(A) Schematic of divergent TrkA-mediated signaling pathways. Molecules that were tested and found to be either required (black), or not required (gray), for maintenance of latency are highlighted. Shc, Src homologous and collagen-like adaptor protein; Grb2, growth factor receptor-bound protein 2; Gab1, Grb2-associated binder-1; PI3-K, phosphatidylinositol 3-kinase; PDK1, phosphoinositide-dependent kinase 1; MEK, mitogen-activated protein kinase (MAPK)/ERK kinase; PLCγ, phospholipase Cγ; Trk, tropomyosin-related kinase receptor, NGF, nerve growth factor. (B) Reactivation assay comparing the response of GFP-HSV-1 infected SCG neurons treated with MEK inhibitor PD98059 (50 μM) to Trk inhibitor K252a (200 nM) or vehicle control DMSO. See also Figure S2 A-C. (C) Reactivation of HSV-1 after 6 d of treatment with the PI3-K inhibitor LY294002 (10 μM), LY303511 (an inactive analogue of LY294002; 10 μM), the Trk inhibitor K252a (200 nM) or DMSO. See also Figure S2 D. (D) Detection of HSV-1 transcripts by RT-PCR. After establishing a non-replicating HSV-1 infection in the presence of NGF and ACV, the ACV was removed and the cultures were treated with LY294002 or DMSO. The RNA was collected 20 h later. Semi-quantitative RT-PCR was used to determine the expression of LAT, ICP27, UL30, UL5 and cellular GAPDH RNA transcripts. (E) Number of GFP+ neurons after 48 h of treatment with LY294002 or vehicle DMSO in the presence of WAY-150138 (20 μg/ml). Ten wells were scored for each condition and data points indicate the number of individual GFP+ cells in a well containing 103 SCG neurons. (F) WAY-150138 prevents HSV-1 spread in SCG neurons. Cultures were infected with HSV-1 Us11-GFP at a MOI of 0.1 in the presence of WAY-150138 or DMSO and the spread of virus visualized by GFP fluorescence after 72 h. (G) Selective inhibition of the PI3-K catalytic subunit p110α leads to reactivation. Infected cultures were treated with the PI3-K inhibitor LY294002, LY303511, PIK75 (PI3-K p110α inhibitor, 0.116 μM), TGX115 (PI3-K p110βδ inhibitor, 2.6 μM), IC87114 (PI3-K p110δ inhibitor, 2.6 μM), or DMSO.
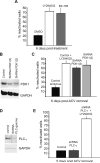
Figure 4. PDK-1 is required in latency
(A) Inhibition of PDK1 induces reactivation. Reactivation in the presence of BX-795 (PDK1 inhibitor, 1 μM), LY294002 or vehicle control DMSO. See also Figure S3. (B) Depletion of PDK1 was verified by immunoblot analysis of lysates prepared after 6 d of infection with each lentivirus. GAPDH served as a loading control. (C) Depletion of PDK1 using shRNAs promotes reactivation. Following ACV removal, GFP-HSV-1 infected cultures were infected with two different lentiviruses expressing an mCherry fluorescent marker and shRNAs against rat PDK1. Cultures infected with a control lentivirus in the presence or absence of LY294002 acted as positive and negative controls respectively. Number of wells expressing GFP was scored after 6 d. (D) Depletion of PLCγ was verified by immunoblot analysis of lysates prepared after 5 d of infection with each lentivirus. GAPDH served as a loading control. (E) Depletion of PLCγ using shRNAs does not induce or prevent reactivation. Following ACV removal, cultures harboring latent GFP-HSV-1 were infected with a control lentivirus or with a lentivirus expressing an shRNA against rat PLCγ. Addition of LY294002 acted as a reactivation control. Number of wells expressing GFP was scored after 5 d.
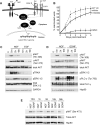
Figure 5. Differential effects of growth factors on HSV-1 reactivation
(A) EGF does not substitute for NGF in maintaining latency. GFP-HSV-1 latently infected SCG neuron cultures were established in the presence of NGF and EGF (50 ng/ml each), washed and then cultured for 5 d in media containing NGF (filled bar), EGF and anti-NGF antibody (shaded bar) and anti-NGF antibody only (open bar). (B) GDNF can partially substitute for NGF. As in A, except that neurons were cultured in media containing NGF and GDNF (50 ng/ml). Neurotrophins were removed by addition of either anti-NGF or anti-GDNF receptor antibodies. (C) and (D) Cultured SCG neurons express functional receptors for EGF and GDNF. Neurons cultures were maintained for 6 h in media lacking growth factors and treated with either EGF or GDNF (50 ng/ml each) and lysates prepared after 5 min, 2 h or 18 h. Levels of activated receptor were detected by immunoblotting using antibodies to phospho-EGFR (C) and phospho-RET, the GDNF receptor (D). Down-regulation of the receptor (shown here at 18 h) after stimulation has been reported previously (Beguinot et al., 1984; Pierchala et al., 2006; Richardson et al., 2006; Stoscheck and Carpenter, 1984).
Figure 6. NGF, EGF, and GDNF activate equivalent downstream pathways in cultured sympathetic neurons but with different kinetics
(A) Sustained RTK-mediated activation of Akt is required to maintain HSV-1 latency. GDNF and EGF signal through the Ret and EGFR tyrosine kinase receptor, respectively and like NGF-TrkA, they activate PI3-K-PDK1-Akt, albeit with different kinetics. The ability of different growth factors to maintain latency is directly proportional to the duration of PI3-K signaling. NGF induces prolonged PI3-K signaling to maintain latency. GDNF has an intermediate ability to sustain the PI3-K pathway and latency. Finally, EGF provides a transient signal and is unable to maintain latency. In black are elements of the pathway tested that maintain latency. PI3-K, phosphatidylinositol 3-kinase; PDK1, phosphoinositide-dependent kinase 1; Trk, tropomyosin-related kinase receptor, NGF, nerve growth factor, Ret, Ret tyrosine kinase receptor; EGFR, epidermal growth factor receptor. (B) Reactivation of latent HSV-1 after treatment with AKT inhibitor VIII (5 mM for 16 h), PI3-K inhibitor LY294002 (10 mM) or DMSO. See also figure S4. (C) and (D) Kinetics of growth factor signaling in SCG neurons. Cultures (4×105 cells/lane) were maintained for 6 h in media lacking growth factors and then supplemented with NGF, EGF or GDNF (50 ng/ml each). Lysates were prepared after 5 min, 2 h or 18 h and analyzed by immunoblotting to detect phosphorylated forms of TrkA, AKT, ERK and PLCγ. NGF treatment was compared to EGF (A) or GDNF (B). GAPDH and Hsp90 serve as loading controls. (E) Profile of Akt phosphorylation by NGF and GDNF. Cultures with supplemented with NGF or GDNF and harvested after 10 min, 1 h, 5 h, 18 h and 36h. Lysates were analyzed by immunoblotting with antibodies against Akt phospho-Ser-473 and total Akt. In the absence of trophic support neurons begin to die at around 36 h as indicated by decreased levels of Hsp90. Note that the NGF 5 h sample is slightly under-loaded.
Comment in
- Keeping HSV‑1 dormant.
David R. David R. Nat Rev Microbiol. 2010 Dec;8(12):838. doi: 10.1038/nrmicro2480. Nat Rev Microbiol. 2010. PMID: 21125701 No abstract available. - Neuroimmunology: Keeping HSV-1 dormant.
David R. David R. Nat Rev Neurosci. 2010 Dec;11(12):790. doi: 10.1038/nrn2953. Nat Rev Neurosci. 2010. PMID: 21132879 No abstract available.
Similar articles
- A primary neuron culture system for the study of herpes simplex virus latency and reactivation.
Kobayashi M, Kim JY, Camarena V, Roehm PC, Chao MV, Wilson AC, Mohr I. Kobayashi M, et al. J Vis Exp. 2012 Apr 2;(62):3823. doi: 10.3791/3823. J Vis Exp. 2012. PMID: 22491318 Free PMC article. - Inducible cyclic AMP early repressor produces reactivation of latent herpes simplex virus type 1 in neurons in vitro.
Colgin MA, Smith RL, Wilcox CL. Colgin MA, et al. J Virol. 2001 Mar;75(6):2912-20. doi: 10.1128/JVI.75.6.2912-2920.2001. J Virol. 2001. PMID: 11222716 Free PMC article. - Remodeling mTORC1 Responsiveness to Amino Acids by the Herpes Simplex Virus UL46 and Us3 Gene Products Supports Replication during Nutrient Insufficiency.
Vink EI, Lee S, Smiley JR, Mohr I. Vink EI, et al. J Virol. 2018 Nov 27;92(24):e01377-18. doi: 10.1128/JVI.01377-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30282708 Free PMC article. - Disturbed Yin-Yang balance: stress increases the susceptibility to primary and recurrent infections of herpes simplex virus type 1.
Yan C, Luo Z, Li W, Li X, Dallmann R, Kurihara H, Li YF, He RR. Yan C, et al. Acta Pharm Sin B. 2020 Mar;10(3):383-398. doi: 10.1016/j.apsb.2019.06.005. Epub 2019 Jun 22. Acta Pharm Sin B. 2020. PMID: 32140387 Free PMC article. Review. - A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.
Kennedy PG, Rovnak J, Badani H, Cohrs RJ. Kennedy PG, et al. J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
Cited by
- c-Jun signaling during initial HSV-1 infection modulates latency to enhance later reactivation in addition to directly promoting the progression to full reactivation.
Dochnal SA, Whitford AL, Francois AK, Krakowiak PA, Cuddy S, Cliffe AR. Dochnal SA, et al. J Virol. 2024 Feb 20;98(2):e0176423. doi: 10.1128/jvi.01764-23. Epub 2024 Jan 9. J Virol. 2024. PMID: 38193709 Free PMC article. - Using Primary SCG Neuron Cultures to Study Molecular Determinants of HSV-1 Latency and Reactivation.
Hu HL, Srinivas KP, Mohr I, Huang TT, Wilson AC. Hu HL, et al. Methods Mol Biol. 2020;2060:263-277. doi: 10.1007/978-1-4939-9814-2_14. Methods Mol Biol. 2020. PMID: 31617183 Free PMC article. - Keeping HSV‑1 dormant.
David R. David R. Nat Rev Microbiol. 2010 Dec;8(12):838. doi: 10.1038/nrmicro2480. Nat Rev Microbiol. 2010. PMID: 21125701 No abstract available. - Differentially expressed genes during spontaneous lytic switch of Marek's disease virus in lymphoblastoid cell lines determined by global gene expression profiling.
Mwangi WN, Vasoya D, Kgosana LB, Watson M, Nair V. Mwangi WN, et al. J Gen Virol. 2017 Apr;98(4):779-790. doi: 10.1099/jgv.0.000744. J Gen Virol. 2017. PMID: 28475033 Free PMC article.
References
- Bartlett SE, Reynolds AJ, Tan T, Heydon K, Hendry IA. Differential mRNA expression and subcellular locations of PI3-kinase isoforms in sympathetic and sensory neurons. J Neurosci Res. 1999;56:44–53. - PubMed
- Berg MM, Sternberg DW, Parada LF, Chao MV. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem. 1992;267:13–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HD023315/HD/NICHD NIH HHS/United States
- NS21072/NS/NINDS NIH HHS/United States
- R01 NS021072/NS/NINDS NIH HHS/United States
- T32 AI007180/AI/NIAID NIH HHS/United States
- R01 GM061139/GM/NIGMS NIH HHS/United States
- S10RR017970/RR/NCRR NIH HHS/United States
- AI073898/AI/NIAID NIH HHS/United States
- GM056927/GM/NIGMS NIH HHS/United States
- R01 AI073898/AI/NIAID NIH HHS/United States
- R56 NS021072/NS/NINDS NIH HHS/United States
- K08 DC009288/DC/NIDCD NIH HHS/United States
- R01 GM056927/GM/NIGMS NIH HHS/United States
- HD23315/HD/NICHD NIH HHS/United States
- GM61139/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous