Circadian regulation of cardiovascular function: a role for vasoactive intestinal peptide - PubMed (original) (raw)
Circadian regulation of cardiovascular function: a role for vasoactive intestinal peptide
Analyne Schroeder et al. Am J Physiol Heart Circ Physiol. 2011 Jan.
Abstract
The circadian system, driven by the suprachiasmatic nucleus (SCN), regulates properties of cardiovascular function. The dysfunction of this timing system can result in cardiac pathology. The neuropeptide vasoactive intestinal peptide (VIP) is crucial for circadian rhythms in a number of biological processes including SCN electrical activity and wheel running behavior. Anatomic evidence indicates that SCN neurons expressing VIP are well positioned to drive circadian regulation of cardiac function through interactions with the autonomic centers. In this study, we tested the hypothesis that loss of VIP would result in circadian deficits in heart rate (HR) and clock gene expression in cardiac tissue. We implanted radiotelemetry devices into VIP-deficient mice and wild-type (WT) controls and continuously recorded HR, body temperature, and cage activity in freely moving mice. Under light-dark conditions, VIP-deficient mice displayed weak rhythms in HR, body temperature, and cage activity, with onsets that were advanced in phase compared with WT mice. Similarly, clock gene expression in cardiac tissue was rhythmic but phase advanced in mutant mice. In constant darkness, the normal circadian rhythms in HR were lost in VIP-deficient mice; however, most mutant mice continued to exhibit circadian rhythms of body temperature with shortened free-running period. The loss of VIP altered, but did not abolish, autonomic regulation of HR. Analysis of the echocardiograms did not find any evidence for a loss of cardiac function in VIP-deficient mice, and the size of the hearts did not differ between genotypes. These results demonstrate that VIP is an important regulator of physiological circadian rhythmicity in the heart.
Figures
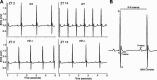
Fig. 1.
In vivo ECG recordings from freely behaving mice. A: representative examples of ECG tracings from wild-type (WT; top) and VIP-deficient (bottom) mice at Zeitgeber (ZT) 2 and ZT 14. B: magnified example of an ECG tracing labeled with features and intervals of the waveform.
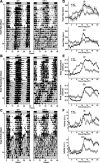
Fig. 2.
Daily pattern of heart rate [HR; in beats/min (bpm)], body temperature, and cage activity [in arbitrary units (AU)] in WT and VIP-deficient mice. Double-plotted raster plots of HR (A), body temperature (B), and cage activity (C) from WT (left) and VIP-deficient mice (right) were created. Mice were trained in 12:12 light-dark (LD) conditions and then placed in constant darkness (DD). Shaded regions indicated when mice were in the dark. Each horizontal row represents a 24-h period that was plotted twice, and each day/row was plotted in succession. Ten days of HR, body temperature, and cage activity were averaged to produce group mean waveforms of HR (D), body temperature (E), and cage activity (F) for WT (n = 7) and VIP-deficient (n = 8) mice under LD (top) and DD (bottom) conditions. All of the WT mice showed strong diurnal and circadian rhythms of HR, body temperature, and cage activity (7 of 7 mice) under LD and DD conditions. VIP-deficient mice displayed dampened diurnal rhythms in HR (6 of 8 mice), body temperature (8 of 8 mice), and cage activity (7 of 8 mice) under LD conditions. Under DD conditions, VIP-deficient mice displayed a dampened circadian rhythm of body temperature (7 of 8 mice) and cage activity (2 of 8 mice). VIP-deficient mice did not display a circadian rhythm of HR under DD conditions (0 of 8 mice), as assessed by periodogram analysis.
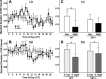
Fig. 3.
Daily pattern of HR variability (HRV) in WT and VIP-deficient mice. HRV was determined by calculating the variance of the time between individual beats, also known as the interbeat interval (IBI). Group mean waveforms of HRV using 10 days of data were produced for WT (n = 7) and VIP-deficient (n = 8) mice under LD (A) and DD (B) conditions. WT mice displayed significant day/night differences under LD (C) and DD (D) conditions. No significant day/night differences were measured for VIP-deficient mice under LD conditions (C), but significance was detected under DD conditions (D). HRV was consistently decreased during the subjective night (S. night) compared with the subjective day (S. day) in both WT and VIP-deficient mice. These data were analyzed using a paired Student's _t_-test (*P < 0.05).
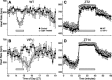
Fig. 4.
Acute regulation of HR in WT and VIP-deficient mice. A: light exposure (1 h) administered at ZT 14 elicited a precipitous drop of HR in WT mice (n = 7). B: VIP-deficient mice (n = 6) responded to the light treatment, but at a rate that was significantly delayed (P < 0.001). Forced exercise triggered the significant increase of HR in WT (n = 5) and VIP-deficient (n = 5) mice at ZT 2 (C) and ZT 14 (D) time points. VIP-deficient mice exhibited an increase in HR that was larger than WT controls. Bars indicate the times of treatment.
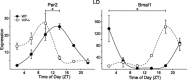
Fig. 5.
Expression profiles of clock genes period 2 (Per2) and Bmal1 in heart tissues of WT and VIP-deficient mice under LD conditions using RT-PCR techniques. Each time point shows mean results of 3–4 hearts. A one-way ANOVA statistical test was used to determine whether there were significant differences in expression. Bars indicate differences in peak expression between WT and VIP-deficient mice; results were analyzed using Student's _t_-test (*P < 0.05).
Similar articles
- Effects of vasoactive intestinal peptide genotype on circadian gene expression in the suprachiasmatic nucleus and peripheral organs.
Loh DH, Dragich JM, Kudo T, Schroeder AM, Nakamura TJ, Waschek JA, Block GD, Colwell CS. Loh DH, et al. J Biol Rhythms. 2011 Jun;26(3):200-9. doi: 10.1177/0748730411401740. J Biol Rhythms. 2011. PMID: 21628547 Free PMC article. - Disrupted reproduction, estrous cycle, and circadian rhythms in female mice deficient in vasoactive intestinal peptide.
Loh DH, Kuljis DA, Azuma L, Wu Y, Truong D, Wang HB, Colwell CS. Loh DH, et al. J Biol Rhythms. 2014 Oct;29(5):355-69. doi: 10.1177/0748730414549767. Epub 2014 Sep 24. J Biol Rhythms. 2014. PMID: 25252712 Free PMC article. - SCN VIP Neurons Are Essential for Normal Light-Mediated Resetting of the Circadian System.
Jones JR, Simon T, Lones L, Herzog ED. Jones JR, et al. J Neurosci. 2018 Sep 12;38(37):7986-7995. doi: 10.1523/JNEUROSCI.1322-18.2018. Epub 2018 Aug 6. J Neurosci. 2018. PMID: 30082421 Free PMC article. - An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei.
Harmar AJ. Harmar AJ. J Neuroendocrinol. 2003 Apr;15(4):335-8. doi: 10.1046/j.1365-2826.2003.01005.x. J Neuroendocrinol. 2003. PMID: 12622830 Review. - The roles of vasoactive intestinal polypeptide in the mammalian circadian clock.
Piggins HD, Cutler DJ. Piggins HD, et al. J Endocrinol. 2003 Apr;177(1):7-15. doi: 10.1677/joe.0.1770007. J Endocrinol. 2003. PMID: 12697032 Review.
Cited by
- Chronic mistimed feeding results in renal fibrosis and disrupted circadian blood pressure rhythms.
Benjamin JI, Pati P, Luong T, Liu X, De Miguel C, Pollock JS, Pollock DM. Benjamin JI, et al. Am J Physiol Renal Physiol. 2024 Nov 1;327(5):F683-F696. doi: 10.1152/ajprenal.00047.2024. Epub 2024 Aug 29. Am J Physiol Renal Physiol. 2024. PMID: 39205662 - The Circadian Biology of Heart Failure.
El Jamal N, Lordan R, Teegarden SL, Grosser T, FitzGerald G. El Jamal N, et al. Circ Res. 2023 Jan 20;132(2):223-237. doi: 10.1161/CIRCRESAHA.122.321369. Epub 2023 Jan 19. Circ Res. 2023. PMID: 36656971 Free PMC article. Review. - Timing of food intake in mice unmasks a role for the cardiomyocyte circadian clock mechanism in limiting QT-interval prolongation.
Schroder EA, Burgess DE, Johnson SR, Ono M, Seward T, Elayi CS, Esser KA, Delisle BP. Schroder EA, et al. Chronobiol Int. 2022 Apr;39(4):525-534. doi: 10.1080/07420528.2021.2011307. Epub 2021 Dec 7. Chronobiol Int. 2022. PMID: 34875962 Free PMC article. - Dissecting the Roles of the Autonomic Nervous System and Physical Activity on Circadian Heart Rate Fluctuations in Mice.
Barazi N, Polidovitch N, Debi R, Yakobov S, Lakin R, Backx PH. Barazi N, et al. Front Physiol. 2021 Oct 18;12:692247. doi: 10.3389/fphys.2021.692247. eCollection 2021. Front Physiol. 2021. PMID: 34733171 Free PMC article. - The Circadian Clock Is Sustained in the Thyroid Gland of VIP Receptor 2 Deficient Mice.
Georg B, Fahrenkrug J, Jørgensen HL, Hannibal J. Georg B, et al. Front Endocrinol (Lausanne). 2021 Sep 1;12:737581. doi: 10.3389/fendo.2021.737581. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34539582 Free PMC article.
References
- Accili EA, Redaelli G, DiFrancesco D. Activation of the hyperpolarization-activated current (If) in sino-atrial node myocytes of the rabbit by vasoactive intestinal peptide. Pflugers Arch 431: 803–805, 1996. - PubMed
- Bechtold DA, Brown TM, Luckman SM, Piggins HD. Metabolic rhythm abnormalities in mice lacking VIP-VPAC2 signaling. Am J Physiol Regul Integr Comp Physiol 294: R344–R351, 2008. - PubMed
- Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED, Young ME. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol 294: H1036–H1047, 2008. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases