In vitro and in vivo effects of polyethylene glycol (PEG)-modified lipid in DOTAP/cholesterol-mediated gene transfection - PubMed (original) (raw)
In vitro and in vivo effects of polyethylene glycol (PEG)-modified lipid in DOTAP/cholesterol-mediated gene transfection
Torben Gjetting et al. Int J Nanomedicine. 2010.
Abstract
Background: DOTAP/cholesterol-based lipoplexes are successfully used for delivery of plasmid DNA in vivo especially to the lungs, although low systemic stability and circulation have been reported. To achieve the aim of discovering the best method for systemic delivery of DNA to disseminated tumors we evaluated the potential of formulating DOTAP/cholesterol lipoplexes with a polyethylene glycol (PEG)-modified lipid, giving the benefit of the shielding and stabilizing properties of PEG in the bloodstream.
Method: A direct comparison of properties in vitro and in vivo of 4 different DOTAP/cholesterol-based lipoplexes containing 0%, 2%, 4%, and 10% PEG was performed using reporter gene activity and radioactive tracer lipid markers to monitor biodistribution.
Results: We found that 10% PEGylation of lipoplexes caused reduced retention in lung and heart tissues of nude mice compared to nonPEGylated lipoplexes, however no significant delivery to xenograft flank tumors was observed. Although PEGylated and nonPEGylated lipoplexes were delivered to cells the ability to mediate successful transfection is hampered upon PEGylation, presumably due to a changed uptake mechanism and intracellular processing.
Conclusion: The eminent in vivo transfection potency of DOTAP/cholesterol-based lipoplexes is well established for expression in lung tumors, but it is unsuitable for expression in non first pass organs such as xenograft flank tumors in mice even after addition of a PEG-lipid in the formulation.
Keywords: DOTAP; biodistribution; gene delivery; lung cancer; polyethylene glycol (PEG); xenograft tumor model.
Figures
Figure 1
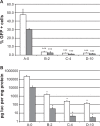
Luciferase reporter gene expression in vivo. After three consecutive daily tail vein injections of DOTAP/chol/DNA lipoplex (A-0*) in nude mice organs were sampled on the fourth day and assayed for luciferase activity, n = 4. Assay background was at 10 pg luc/g protein (indicated with dotted line).
Figure 2
Agarose gel electrophoresis/ethidium bromide staining of DNA/lipoplexes with different content of PEG-lipid compared to the migration of free plasmid DNA (free DNA) and a DNA size marker.
Figure 3
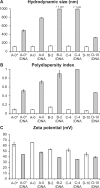
Physical characterization of lipoparticles using a Zetasizer. Each of the four liposome formulations was analyzed before (white columns) and after (grey columns) mixing with plasmid DNA. A) The hydrodynamic size. B) The polydispersity index. C) The zeta potential of the particles. The estimate and the standard error are given for each measurement. Three independent experiments yielded equivalent results.
Figure 4
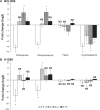
Reporter gene expression by transfection of NCI-H69 (SCLC) and H1299 (NSCLC) cells in vitro. A) EGFP fluorescence in single cells was measured by FACS flow cytometry and expressed as the percentage of EGFP positive cells. *** indicates a significant difference from A-0 (P < 0.001). B) Luciferase activity was measured in cell lysates and expressed as picogram luciferase per milligram of protein. Data from four independent experiments with two different lipid preparations were collected and the average and standard error of the mean are given. *indicates a significant difference from A-0 (P < 0.05).
Figure 5
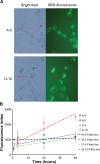
Cellular uptake of fluorescently labeled lipoplexes. A) Bright-field and fluorescence microscope pictures taken two days after H1299 cells were transfected with NBD-labeled A-0 (upper panel) and D-10 lipoplexes (lower panel). Scale bar marked in red indicates 15 μm. B) NBD fluorescence in cell lysates was measured at different time points after addition to cell cultures. Non-PEGylated A-0 lipoplex is rapidly taken up by cells to a saturating level within one hour and remain constant hereafter (fitted line [estimate ± standard error]: Fluorescence index = (1001 ± 102) + (2.8 ± 3.6) × time) Slope confidence interval includes 0. In contrast, PEGylated lipoplexes are gradually taken up over two days. Fitted lines: B-2: Fluorescence index = (626 ± 115) + (15 ± 4.3) x time; C-4: Fluorescence index = (487 ± 71) + (15 ± 2.9) × time; D-10: Fluorescence index = (678 ± 92) + (44 ± 3.7) × time. Slope confidence interval exclude 0. Data are normalized and averaged from triplicates of three independent experiments (average ± SD).
Figure 6
Effects of endocytosis inhibitors on luciferase reporter activity measured in (A) NCI-H69 and (B) 1299 cells. Cells were incubated one hour prior to transfection and then exposed to inhibitors for the entire incubation (48 hours). Chloroquine (20 μM (NCI-H69), 30 μM (H1299)), chlorpromazine 10 μM (NCI-H69), 20 μM (H1299), filipin (4 μM), and cytochalasin B (25 μM). Data from 3–5 independent experiments were pooled and log2 transformed to obtain fold change in expression. Error bars indicate standard error of the mean, *means that 0 is not included in confidence interval (P < 0.05), no change: N.S.
Figure 7
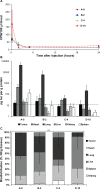
A) Blood availability of tritium-labeled lipoplexes with PEGylation. Following tail vein injection of lipoplexes blood samples were drawn by periobital plexus puncture (eye vein blood) after 15 minutes, 2 hours, 5 hours, and 24 hours, and the amount of radioactivity was quantified by scintillation counting. B) Biodistribution of luciferase activity. Mice injected with tritium-labeled DNA/lipoplex with different degrees of PEGylation were sacrificed after 24 hours and samples from organs were analyzed for luciferase activity. Background level in assay is 10 pg/g protein (indicated with dotted line). C) Biodistribution of radioactivity. Tritium counts were expressed as CPM per gram organ sample weight (CPM/g) and the relative distribution in tumor, heart, lung, liver, kidney, and spleen was calculated. Error bars indicate standard deviations. In each group, n = 3–5. A two sided _t_-test of accumulation in heart/lung samples between A-0 and D-10 results in P = 1.9E-05 (***).
Similar articles
- siRNA delivery to lung-metastasized tumor by systemic injection with cationic liposomes.
Hattori Y, Nakamura A, Arai S, Kawano K, Maitani Y, Yonemochi E. Hattori Y, et al. J Liposome Res. 2015;25(4):279-86. doi: 10.3109/08982104.2014.992024. Epub 2015 Sep 4. J Liposome Res. 2015. PMID: 25543847 - Cholesterol-rich lipid-mediated nanoparticles boost of transfection efficiency, utilized for gene editing by CRISPR-Cas9.
Hosseini ES, Nikkhah M, Hosseinkhani S. Hosseini ES, et al. Int J Nanomedicine. 2019 Jun 11;14:4353-4366. doi: 10.2147/IJN.S199104. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31354265 Free PMC article. - Pegylated GL67 lipoplexes retain their gene transfection activity after exposure to components of CF mucus.
Sanders NN, De Smedt SC, Cheng SH, Demeester J. Sanders NN, et al. Gene Ther. 2002 Mar;9(6):363-71. doi: 10.1038/sj.gt.3301663. Gene Ther. 2002. PMID: 11960312 - Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.
Hadinoto K, Sundaresan A, Cheow WS. Hadinoto K, et al. Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review. - DOTAP (and other cationic lipids): chemistry, biophysics, and transfection.
Simberg D, Weisman S, Talmon Y, Barenholz Y. Simberg D, et al. Crit Rev Ther Drug Carrier Syst. 2004;21(4):257-317. doi: 10.1615/critrevtherdrugcarriersyst.v21.i4.10. Crit Rev Ther Drug Carrier Syst. 2004. PMID: 15638468 Review.
Cited by
- Investigation of the adsorption behavior of BSA with tethered lipid layer-modified solid-state nanopores in a wide pH range.
Tian H, Xie W, He S, Zhou D, Fang S, Liang L, Wang D. Tian H, et al. RSC Adv. 2019 May 17;9(27):15431-15436. doi: 10.1039/c9ra00698b. eCollection 2019 May 14. RSC Adv. 2019. PMID: 35514824 Free PMC article. - Assessment of in-vivo biocompatibility evaluation of phytogenic gold nanoparticles on Wistar albino male rats.
Pannerselvam B, Devanathadesikan V, Alagumuthu TS, Kanth SV, Pudupalayam Thangavelu K. Pannerselvam B, et al. IET Nanobiotechnol. 2020 Jun;14(4):314-324. doi: 10.1049/iet-nbt.2019.0116. IET Nanobiotechnol. 2020. PMID: 32463022 Free PMC article. - Size-dependent in vivo toxicity of PEG-coated gold nanoparticles.
Zhang XD, Wu D, Shen X, Liu PX, Yang N, Zhao B, Zhang H, Sun YM, Zhang LA, Fan FY. Zhang XD, et al. Int J Nanomedicine. 2011;6:2071-81. doi: 10.2147/IJN.S21657. Epub 2011 Sep 20. Int J Nanomedicine. 2011. PMID: 21976982 Free PMC article. - Investigation of enzyme-sensitive lipid nanoparticles for delivery of siRNA to blood-brain barrier and glioma cells.
Bruun J, Larsen TB, Jølck RI, Eliasen R, Holm R, Gjetting T, Andresen TL. Bruun J, et al. Int J Nanomedicine. 2015 Sep 24;10:5995-6008. doi: 10.2147/IJN.S87334. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26451106 Free PMC article. - Development, Characterization and Use of Liposomes as Amphipathic Transporters of Bioactive Compounds for Melanoma Treatment and Reduction of Skin Inflammation: A Review.
Castañeda-Reyes ED, Perea-Flores MJ, Davila-Ortiz G, Lee Y, Gonzalez de Mejia E. Castañeda-Reyes ED, et al. Int J Nanomedicine. 2020 Oct 8;15:7627-7650. doi: 10.2147/IJN.S263516. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33116492 Free PMC article. Review.
References
- Kang HC, Lee M, Bae YH. Polymeric gene carriers. Crit Rev Eukaryot Gene Expr. 2005;15:317–342. - PubMed
- Kawano T, Okuda T, Aoyagi H, Niidome T. Long circulation of intravenously administered plasmid DNA delivered with dendritic poly(L-lysine) in the blood flow. J Control Release. 2004;99:329–337. - PubMed
- Ewert KK, Ahmad A, Bouxsein NF, Evans HM, Safinya CR. Non-viral gene delivery with cationic liposome-DNA complexes. Methods Mol Biol. 2008;433:159–175. - PubMed
- Safinya CR, Ewert K, Ahmad A, et al. Cationic liposome-DNA complexes: from liquid crystal science to gene delivery applications. Philos Transact A Math Phys Eng Sci. 2006;364:2573–2596. - PubMed
- Templeton NS, Lasic DD, Frederik PM, Strey HH, Roberts DD, Pavlakis GN. Improved DNA: liposome complexes for increased systemic delivery and gene expression. Nat Biotechnol. 1997;15:647–652. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical