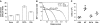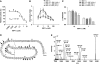Polymerization of MIP-1 chemokine (CCL3 and CCL4) and clearance of MIP-1 by insulin-degrading enzyme - PubMed (original) (raw)
. 2010 Dec 1;29(23):3952-66.
doi: 10.1038/emboj.2010.256. Epub 2010 Oct 19.
Qing Guo, Liang Guo, Martin Lenz, Feng Qian, Rory R Koenen, Hua Xu, Alexander B Schilling, Christian Weber, Richard D Ye, Aaron R Dinner, Wei-Jen Tang
Affiliations
- PMID: 20959807
- PMCID: PMC3020635
- DOI: 10.1038/emboj.2010.256
Polymerization of MIP-1 chemokine (CCL3 and CCL4) and clearance of MIP-1 by insulin-degrading enzyme
Min Ren et al. EMBO J. 2010.
Abstract
Macrophage inflammatory protein-1 (MIP-1), MIP-1α (CCL3) and MIP-1β (CCL4) are chemokines crucial for immune responses towards infection and inflammation. Both MIP-1α and MIP-1β form high-molecular-weight aggregates. Our crystal structures reveal that MIP-1 aggregation is a polymerization process and human MIP-1α and MIP-1β form rod-shaped, double-helical polymers. Biophysical analyses and mathematical modelling show that MIP-1 reversibly forms a polydisperse distribution of rod-shaped polymers in solution. Polymerization buries receptor-binding sites of MIP-1α, thus depolymerization mutations enhance MIP-1α to arrest monocytes onto activated human endothelium. However, same depolymerization mutations render MIP-1α ineffective in mouse peritoneal cell recruitment. Mathematical modelling reveals that, for a long-range chemotaxis of MIP-1, polymerization could protect MIP-1 from proteases that selectively degrade monomeric MIP-1. Insulin-degrading enzyme (IDE) is identified as such a protease and decreased expression of IDE leads to elevated MIP-1 levels in microglial cells. Our structural and proteomic studies offer a molecular basis for selective degradation of MIP-1. The regulated MIP-1 polymerization and selective inactivation of MIP-1 monomers by IDE could aid in controlling the MIP-1 chemotactic gradient for immune surveillance.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
Structure of MIP-1α and MIP-1β polymer. (A) Sequence alignment of MIP-1α and MIP-1β with different residues highlighted in blue. (B) Crystal structure of MIP-1α polymer. A 40mer is depicted to show the periodicity of two 360° turns, and 20 dimers within the 40mer are labelled. The C-terminal helix of each dimer pair is coloured in red and green, respectively. (C) Secondary structure and (D) surface charge comparison of the MIP-1α and MIP-1β decamer. The molecular surface is coloured from negative (red, −6kT) to positve (blue, −6kT) as calculated by APBS (38). (E) Structural comparison of MIP-1 X-ray structure (solid colour) from this study with NMR structures of MIP-1α and MIP-1β (transparent colour). Structure comparison of MIP-1 monomer is on the left and that of dimer is on the right. The PDB code for MIP-1α and MIP-1β NMR structures are 1B50 and 1HUN, respectively. (F) Charge complementarity between MIP-1 dimers. The molecular surface is coloured as calculated by APBS (<−6kT in red, 0kT in white, and >+6kT in blue). (G) Detailed interaction of MIP-1α at the dimer–dimer interface formed by the interaction of monomers diagonal to each other (top right panel) and between the side-by-side monomers (bottom right panel).
Figure 2
Characterization and structure analysis of MIP-1α (D27A) polymer. (A) Overall structure of MIP-1α D27A polymer, which consists of the MIP-1α D27A polymer (coloured in red and green on their C-terminal α-helices) and MIP-1α D27A octamer. Comparison of MIP-1α D27A polymer and octamer either as tetramer (B) or octamer (C), revealing the difference in their degree of rotation. (D) Comparison of MIP-1α D27A polymer and octamer based on their thermal B factor. (E) Comparison of two dimers within the MIP-1α D27A octamer, revealing the conformational change in the C-terminal α-helices in one of the four dimers within the MIP-1α D27A octamer.
Figure 3
Characterization of MIP-1α and MIP-1β polymers in solution. (A) SEC of 1 mg/ml MIP-1α, MIP-1β and MIP-1α D27A with or without 0.1 mg/ml heparin. SEC was performed using phosphate-buffered saline (PBS). (B) Experimental scattering curves for 1 mg/ml MIP-1α, MIP-1β and MIP-1α D27A with or without 0.1 mg/ml heparin in PBS. Insets: cross-section, Guinier rod plots. (C) Schematic representation of our model for the polymerization process. The detailed mathematical modelling is appended in the Supplementary Material. (D) Fitting of the experimental data (solid line) with the calculated scattering from either the best polymer distribution given by our model (dashed line) or the best single polymer (dotted line) with difference between the experimental and fitted theoretical curves shown on bottom. Inset: size distribution of the polymers length distribution. The fitting errors _I_0 × I_theo_a(q)−_I_exp(q)+_I_b are represented in the deviation curve. Parameter values were chosen to minimize the fit function  , as described in the Supplementary Material.
, as described in the Supplementary Material.
Figure 4
Effects of MIP-1 depolymerization mutations on biological functions of MIP-1. (A) Arrest of PBMCs onto TNFα-activated HUVECs under flow conditions after preincubation without or with MIP-1α WT or D27A or P8A (1 μg/ml). The _P_-values for MIP-1α WT versus D27A or P8A are <0.02. (B) Theoretical model for the MIP-1 monomer profile away from its secretion site in the presence of diffusion and degradation by IDE. Three curves are plotted, corresponding to three different set of chemical reactions: degradation only (solid line; M (monomer only)); degradation and dimerization (dashed line; M+D); degradation, dimerization and polymerization of the dimer (dotted line; M+D+P). The arrow marks the cutoff point, where the concentration drop across the cell is larger than 1% on the M+D+P gradient so that effective chemotaxis occurs far away from the source of MIP-1 (where it is steep), but not in its vicinity (where it plateaus). The parameters of the model are chosen as follows: diffusion constant: 1 μm2/s; degradation rate: 10−3/s; MIP-1 secretion rate at the secretion site: 2 × 10−11 mol/m2/s; dimerization rate: 106/M/s; dimer disassembly rate: 10−2/s; dimer–dimer binding rate: 105/M/s; dimer–dimer unbinding rate: 10−2/s. The details in this mathematical modelling are appended in the Supplementary Material. (C) Peritoneal cell accumulations in response to wild-type MIP-1α, MIP-1α D27A or MIP-1α P8A. Male C57BL/6 mice were intraperitoneally injected with 10 μg of either MIP-1α WT or D27A or P8A mutant for 20 h. Cells accumulated in the peritoneal cavity were collected and counted. Values shown are means±s.e.m. of measurements using five mice per group. The _P_-values for the comparison of MIP-1α WT with PBS and MIP-1α D27A mutant are <0.0001 and that with MIP-1α WT and P8A mutant is 0.0012.
Figure 5
Identification of MIP-1α as a high-affinity substrate for IDE. (A) MIP-1α/MIP-1β/RANTES are modelled into the catalytic chamber of IDE. IDE is depicted as light green and the chamber as dark green. Molecular surface of MIP-1α/MIP-1β/RANTES is coloured from negative (red, −6kT) to positive (blue, +6kT) as calculated by APBS (38). (B) Representative MS spectra of MIP-1α WT (top), D27A and MIP-1β (middle), RANTES (bottom) with and without IDE. MIP-1α WT and D27A proteins (8 μM) alone or digested by IDE (0.16 μM) with or without the presence of heparin at 37°C for 30 s were analysed by MALDI-TOF MS. MIP-1β and RANTES samples were treated similarly at 37°C for 15 min. (C) IDE expression of BV-2 stable lines encoding shRNA against mouse IDE. The total cell lysates of BV-2 cells that expressed the control shRNA or IDE shRNA were immunoblotted with anti-IDE (top panel) and anti-ERK1/2 (bottom panel). (D) The secretion of MIP-1 and RANTES by BV-2 cells that have reduced IDE expression. Secreted MIP-1 chemokine levels from IDE knockdown BV-2 cells with or without 10 ng/ml LPS stimulation for 3 h were determined by ELISA assays.
Figure 6
Functional analyses on the effect of MIP-1 degradation by IDE. (A) Inhibition of IDE-mediated degradation of substrate V by MIP-1α. IDE activity on substrate V was measured at 37°C in the presence of indicated concentrations of MIP-1α. (B, C) Effects of IDE on the chemotaxis and increase of [Ca2+]i of MIP-1α on THP-1 Cells. MIP-1α was preincubated with IDE at the indicated molar ratio at 37°C for 15 min. For the chemotactic activity (B), chemotactic response was expressed as mean chemotaxis index. For the MIP-1-mediated increase of [Ca2+]i (C), fura-2 loaded THP-1 cells were stimulated with either 1 or 10 nM MIP-1α as the positive control or the same concentrations of MIP-1α that were pretreated with IDE (MIP-1α+IDE). MIP-1α-dependent increases of [Ca2+]i were monitored by ratio of fluorescence at 340 over 380 nm. The change of [Ca2+]i is indicated by ΔF340/F380, which is the difference between the peak F340/F380 value after addition of MIP-1α and the basal level before stimulation. (D) Summary of IDE cleavage sites on the MIP-1α primary sequence by mass spectrometry analysis is illustrated. Initial cleavage sites and secondary cutting sites are shown as big and small arrows, respectively. (E) Representative ESI-FTICR-MS spectrum of MIP-1α after a 2-min IDE digestion.
Figure 7
Structural analyses on MIP-1α-bound IDE. (A) Overall secondary structure of IDE-CF-E111Q in complex with MIP-1α. N- and C-terminal domains of IDE are coloured cyan and grey, respectively. MIP-1α is coloured orange. (B) The detailed interaction of MIP-1α at the exosite (left) and catalytic site (right). Composite omit map was coloured orange at 1σ level; the atoms oxygen, nitrogen and carbon of substrates are shown in red, blue and orange, respectively. (C) Comparison of MIP-1α in the free form (1B50, green) with IDE-bound form (3H44, red). The corresponding regions of MIP-1α found in IDE-bound form are coloured as red. (D) The three-dimensional structure of MIP-1α (2X69). The residues for IDE initial cleavage sites and secondary sites are coloured red and light green, respectively. Residues involved in receptor CCR5 binding are highlighted yellow. (E) Structural comparison of IDE in complex with Aβ (2G47), insulin (2WBY), bradykinin (3CWW) and MIP-1α (3H44). IDE and its substrates are coloured green and red, respectively.
Similar articles
- Structures of human CCL18, CCL3, and CCL4 reveal molecular determinants for quaternary structures and sensitivity to insulin-degrading enzyme.
Liang WG, Ren M, Zhao F, Tang WJ. Liang WG, et al. J Mol Biol. 2015 Mar 27;427(6 Pt B):1345-1358. doi: 10.1016/j.jmb.2015.01.012. Epub 2015 Jan 28. J Mol Biol. 2015. PMID: 25636406 Free PMC article. - MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4.
Ramos CD, Canetti C, Souto JT, Silva JS, Hogaboam CM, Ferreira SH, Cunha FQ. Ramos CD, et al. J Leukoc Biol. 2005 Jul;78(1):167-77. doi: 10.1189/jlb.0404237. Epub 2005 Apr 14. J Leukoc Biol. 2005. PMID: 15831559 - Identification of amino acid residues critical for aggregation of human CC chemokines macrophage inflammatory protein (MIP)-1alpha, MIP-1beta, and RANTES. Characterization of active disaggregated chemokine variants.
Czaplewski LG, McKeating J, Craven CJ, Higgins LD, Appay V, Brown A, Dudgeon T, Howard LA, Meyers T, Owen J, Palan SR, Tan P, Wilson G, Woods NR, Heyworth CM, Lord BI, Brotherton D, Christison R, Craig S, Cribbes S, Edwards RM, Evans SJ, Gilbert R, Morgan P, Randle E, Schofield N, Varley PG, Fisher J, Waltho JP, Hunter MG. Czaplewski LG, et al. J Biol Chem. 1999 Jun 4;274(23):16077-84. doi: 10.1074/jbc.274.23.16077. J Biol Chem. 1999. PMID: 10347159 - Macrophage inflammatory protein-1.
Menten P, Wuyts A, Van Damme J. Menten P, et al. Cytokine Growth Factor Rev. 2002 Dec;13(6):455-81. doi: 10.1016/s1359-6101(02)00045-x. Cytokine Growth Factor Rev. 2002. PMID: 12401480 Review. - Macrophage inflammatory protein-1.
Maurer M, von Stebut E. Maurer M, et al. Int J Biochem Cell Biol. 2004 Oct;36(10):1882-6. doi: 10.1016/j.biocel.2003.10.019. Int J Biochem Cell Biol. 2004. PMID: 15203102 Review.
Cited by
- Multiple cytokine analysis of aqueous humor in uveitis with or without secondary glaucoma.
Xiao J, Zhao C, Cheng G, Song H, Zhang Y, Zhang M. Xiao J, et al. BMC Ophthalmol. 2024 Oct 15;24(1):451. doi: 10.1186/s12886-024-03691-4. BMC Ophthalmol. 2024. PMID: 39407182 Free PMC article. - The Impact of the Reduction in Environmental Pollution during COVID-19 Lockdown on Healthy Individuals.
Romero-Mesones C, de Homdedeu M, Soler-Segovia D, Gómez-Ollés C, Espejo-Castellanos D, Ojanguren I, Saez-Gimenez B, Cruz MJ, Munoz X. Romero-Mesones C, et al. Toxics. 2024 Jul 4;12(7):492. doi: 10.3390/toxics12070492. Toxics. 2024. PMID: 39058144 Free PMC article. - Deciphering the Role of Epstein-Barr Virus Latent Membrane Protein 1 in Immune Modulation: A Multifaced Signalling Perspective.
Šimičić P, Batović M, Stojanović Marković A, Židovec-Lepej S. Šimičić P, et al. Viruses. 2024 Apr 4;16(4):564. doi: 10.3390/v16040564. Viruses. 2024. PMID: 38675906 Free PMC article. Review. - Protective effect of PDE4B subtype-specific inhibition in an App knock-in mouse model for Alzheimer's disease.
Armstrong P, Güngör H, Anongjanya P, Tweedy C, Parkin E, Johnston J, Carr IM, Dawson N, Clapcote SJ. Armstrong P, et al. Neuropsychopharmacology. 2024 Sep;49(10):1559-1568. doi: 10.1038/s41386-024-01852-z. Epub 2024 Mar 23. Neuropsychopharmacology. 2024. PMID: 38521860 Free PMC article. - scRNA-seq analysis discovered suppression of immunomodulatory dependent inflammatory response in PMBCs exposed to silver nanoparticles.
Perumalsamy H, Xiao X, Kim HY, Yoon TH. Perumalsamy H, et al. J Nanobiotechnology. 2024 Mar 17;22(1):118. doi: 10.1186/s12951-024-02364-0. J Nanobiotechnology. 2024. PMID: 38494495 Free PMC article.
References
- Allen SJ, Crown SE, Handel TM (2007) Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol 25: 787–820 - PubMed
- Baltus T, Weber KSC, Johnson Z, Proudfoot AEI, Weber C (2003) Oligomerization of RANTES is required for CCR1-mediated arrest but not CCR5-mediated transmigration of leukocytes on inflamed endothelium. Blood 102: 1985–1988 - PubMed
- Bernstein SH, Eaves CJ, Herzig R, Fay J, Lynch J, Phillips GL, Christiansen N, Reece D, Ericson S, Stephan M, Kovalsky M, Hawkins K, Rasmussen H, Devos A, Herzig GP (1997) A randomized phase II study of BB-10010: a variant of human macrophage inflammatory protein-1alpha for patients receiving high-dose etoposide and cyclophosphamide for malignant lymphoma and breast cancer. Br J Haematol 99: 888–895 - PubMed
- Bishop JR, Schuksz M, Esko JD (2007) Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446: 1030–1037 - PubMed
- Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P (1995) Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270: 1811–1815 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM081539-04/GM/NIGMS NIH HHS/United States
- R01 GM081539/GM/NIGMS NIH HHS/United States
- R01 GM081539-02/GM/NIGMS NIH HHS/United States
- R01 GM081539-03/GM/NIGMS NIH HHS/United States
- GM81539/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials