A phase 1-2 study of a farnesyltransferase inhibitor, tipifarnib, combined with idarubicin and cytarabine for patients with newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndrome - PubMed (original) (raw)
Clinical Trial
. 2011 Mar 15;117(6):1236-44.
doi: 10.1002/cncr.25575. Epub 2010 Oct 19.
Affiliations
- PMID: 20960519
- PMCID: PMC4061136
- DOI: 10.1002/cncr.25575
Clinical Trial
A phase 1-2 study of a farnesyltransferase inhibitor, tipifarnib, combined with idarubicin and cytarabine for patients with newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndrome
Elias Jabbour et al. Cancer. 2011.
Abstract
Background: The authors conducted a phase 1/2 study of tipifarnib in combination with idarubicin and cytarabine (IA) in 95 patients with previously untreated acute myeloid leukemia (AML) or high-risk myelodysplastic syndrome.
Methods: Induction consisted of idarubicin 12 mg/m(2) a day on days 1-3, cytarabine 1.5 g/m(2) intravenously continuously daily on days 1-4 (days 1-3 if age ≥60 years), and tipifarnib, with the first cohort (n = 6) receiving 200 mg orally twice a day and all others receiving 300 mg twice a day for 21 days every 28 days. Consolidation consisted of 5 courses of idarubicin 8 mg/m(2) a day on days 1-2, cytarabine 0.75 g/m(2) a day on days 1-3, and tipifarnib 300 mg twice a day for 14 days every 4-6 weeks. Maintenance with tipifarnib 300 mg twice a day for 21 days every 4-6 weeks was continued for 6 months.
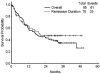
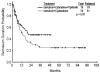
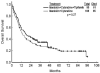
Results: With a median follow-up of 33 months, 61 patients achieved complete remission (CR) (64%), and 9 achieved complete remission with incomplete platelet recovery (CRp) (9%). The median duration of CR was not reached. Median overall survival was 17 months. The most common grade 3 adverse events were gastrointestinal toxicities, liver dysfunction, and skin rash. Compared with historical IA, IA and tipifarnib showed a better CR duration (P = .04) and a trend toward a higher CR rate in patients with chromosome 5/7 abnormalities.
Conclusions: The combination of IA and tipifarnib is safe and active. Further studies exploring different dosages and schedules are warranted, particularly in patients with poor-risk AML.
Copyright © 2010 American Cancer Society.
Figures
Figure 1
Survival and remission duration are shown.
Figure 2
Remission duration with idarubicin and cytarabine versus with idarubicin, cytarabine, and tipifarnib (including patients in CR and CRp) is shown.
Figure 3
Overall survival idarubicin and cytarabine versus with idarubicin, cytarabine, and tipifarnib is shown.
Similar articles
- Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a single-arm, phase 2 study.
Ravandi F, Assi R, Daver N, Benton CB, Kadia T, Thompson PA, Borthakur G, Alvarado Y, Jabbour EJ, Konopleva M, Takahashi K, Kornblau S, DiNardo CD, Estrov Z, Flores W, Basu S, Allison J, Sharma P, Pierce S, Pike A, Cortes JE, Garcia-Manero G, Kantarjian HM. Ravandi F, et al. Lancet Haematol. 2019 Sep;6(9):e480-e488. doi: 10.1016/S2352-3026(19)30114-0. Epub 2019 Aug 7. Lancet Haematol. 2019. PMID: 31400961 Free PMC article. Clinical Trial. - Venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a cohort from a single-centre, single-arm, phase 2 trial.
Kadia TM, Reville PK, Borthakur G, Yilmaz M, Kornblau S, Alvarado Y, Dinardo CD, Daver N, Jain N, Pemmaraju N, Short N, Wang SA, Tidwell RSS, Islam R, Konopleva M, Garcia-Manero G, Ravandi F, Kantarjian HM. Kadia TM, et al. Lancet Haematol. 2021 Aug;8(8):e552-e561. doi: 10.1016/S2352-3026(21)00192-7. Lancet Haematol. 2021. PMID: 34329576 Free PMC article. Clinical Trial. - Epigenetic priming with decitabine followed by low dose idarubicin and cytarabine in acute myeloid leukemia evolving from myelodysplastic syndromes and higher-risk myelodysplastic syndromes: a prospective multicenter single-arm trial.
Zhou X, Mei C, Zhang J, Lu Y, Lan J, Lin S, Zhang Y, Kuang Y, Ren Y, Ma L, Wei J, Ye L, Xu W, Li K, Lu C, Jin J, Tong H. Zhou X, et al. Hematol Oncol. 2020 Oct;38(4):531-540. doi: 10.1002/hon.2755. Epub 2020 Jun 24. Hematol Oncol. 2020. PMID: 32469434 - High-dose cytarabine (24 g/m2) in combination with idarubicin (HiDAC-3) results in high first-cycle response with limited gastrointestinal toxicity in adult acute myeloid leukaemia.
Low M, Lee D, Coutsouvelis J, Patil S, Opat S, Walker P, Schwarer A, Salem H, Avery S, Spencer A, Wei A. Low M, et al. Intern Med J. 2013 Mar;43(3):294-7. doi: 10.1111/j.1445-5994.2012.02868.x. Intern Med J. 2013. PMID: 22757980 Review. - Remission induction in acute myeloid leukemia.
Stein EM, Tallman MS. Stein EM, et al. Int J Hematol. 2012 Aug;96(2):164-70. doi: 10.1007/s12185-012-1121-y. Epub 2012 Jul 13. Int J Hematol. 2012. PMID: 22791508 Free PMC article. Review.
Cited by
- Chronic myelomonocytic leukemia: Forefront of the field in 2015.
Benton CB, Nazha A, Pemmaraju N, Garcia-Manero G. Benton CB, et al. Crit Rev Oncol Hematol. 2015 Aug;95(2):222-42. doi: 10.1016/j.critrevonc.2015.03.002. Epub 2015 Mar 14. Crit Rev Oncol Hematol. 2015. PMID: 25869097 Free PMC article. Review. - Small molecule inhibitors in acute myeloid leukemia: from the bench to the clinic.
Al-Hussaini M, DiPersio JF. Al-Hussaini M, et al. Expert Rev Hematol. 2014 Aug;7(4):439-64. doi: 10.1586/17474086.2014.932687. Expert Rev Hematol. 2014. PMID: 25025370 Free PMC article. Review. - Tipifarnib prevents development of hypoxia-induced pulmonary hypertension.
Duluc L, Ahmetaj-Shala B, Mitchell J, Abdul-Salam VB, Mahomed AS, Aldabbous L, Oliver E, Iannone L, Dubois OD, Storck EM, Tate EW, Zhao L, Wilkins MR, Wojciak-Stothard B. Duluc L, et al. Cardiovasc Res. 2017 Mar 1;113(3):276-287. doi: 10.1093/cvr/cvw258. Cardiovasc Res. 2017. PMID: 28395021 Free PMC article. - Current therapy of myelodysplastic syndromes.
Zeidan AM, Linhares Y, Gore SD. Zeidan AM, et al. Blood Rev. 2013 Sep;27(5):243-59. doi: 10.1016/j.blre.2013.07.003. Epub 2013 Jul 27. Blood Rev. 2013. PMID: 23954262 Free PMC article. Review. - "Tip" the Scale of Cardiac Repair via Reducing Pathological Extracellular Vesicles.
Yue Z, Cheng K. Yue Z, et al. Circ Res. 2024 Jul 5;135(2):298-300. doi: 10.1161/CIRCRESAHA.124.324955. Epub 2024 Jul 4. Circ Res. 2024. PMID: 38963869 No abstract available.
References
- Estey E. Treatment of relapsed and refractory acute myeloid leukemia. Leukemia. 2000;14:476–479. - PubMed
- Leopold LH, Willemze R. The treatment of acute myeloid leukemia in first relapse: a comprehensive review of the literature. Leuk Lymphoma. 2002;43:1715–1727. - PubMed
- Ravandi F, Kantarjian H, Giles F, Cortes J. New agents in acute myeloid leukemia and other myeloid disorders. Cancer. 2004;100:441–454. - PubMed
- Fenaux P, Mufti G, Santini V, et al. Azacitidine (AZA) treatment prolongs overall survival (OS) in higher-risk MDS patients compared with conventional cure regimens (CCR): results of the AZA-001 phase III study. Blood. 2007;110:250a. Abstract 817.
- Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous