Circular logic: nonribosomal peptide-like macrocyclization with a ribosomal peptide catalyst - PubMed (original) (raw)
Circular logic: nonribosomal peptide-like macrocyclization with a ribosomal peptide catalyst
John A McIntosh et al. J Am Chem Soc. 2010.
Abstract
A protease from ribosomal peptide biosynthesis macrocyclizes diverse substrates, including those resembling nonribosomal peptide and hybrid polyketide-peptide products. The proposed mechanism is analogous to thioesterase-catalyzed chemistry, but the substrates are amide bonds rather than thioesters.
Figures
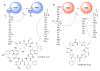
Figure 1. Macrocyclization in ribosomal and nonribosomal synthesis
A) The nonribosomal TycC TE domain circularizes tyrocidines. PCP = peptidyl carrier protein. B) The ribosomally acting PatG protease circularizes patellamides and many other compounds. The proposed catalytic mechanism is indicated here. Tzl = thiazol(ine); MOzl = methyloxazoline.
Figure 2. Products of PatG macrocyclization
A) Representative known natural products cyclized by PatG, out of a total of 39 known natural products in this series. B) Macrocyclization products from this study, showing side-chain circularization (28, 43) and polyketide-like cyclization (41, 42).
Similar articles
- The thioesterase domain from a nonribosomal peptide synthetase as a cyclization catalyst for integrin binding peptides.
Kohli RM, Takagi J, Walsh CT. Kohli RM, et al. Proc Natl Acad Sci U S A. 2002 Feb 5;99(3):1247-52. doi: 10.1073/pnas.251668398. Epub 2002 Jan 22. Proc Natl Acad Sci U S A. 2002. PMID: 11805307 Free PMC article. - Generality of peptide cyclization catalyzed by isolated thioesterase domains of nonribosomal peptide synthetases.
Kohli RM, Trauger JW, Schwarzer D, Marahiel MA, Walsh CT. Kohli RM, et al. Biochemistry. 2001 Jun 19;40(24):7099-108. doi: 10.1021/bi010036j. Biochemistry. 2001. PMID: 11401555 - Macrocyclization strategies in polyketide and nonribosomal peptide biosynthesis.
Kopp F, Marahiel MA. Kopp F, et al. Nat Prod Rep. 2007 Aug;24(4):735-49. doi: 10.1039/b613652b. Epub 2007 May 10. Nat Prod Rep. 2007. PMID: 17653357 Review. - Chemoenzymatic route to macrocyclic hybrid peptide/polyketide-like molecules.
Kohli RM, Burke MD, Tao J, Walsh CT. Kohli RM, et al. J Am Chem Soc. 2003 Jun 18;125(24):7160-1. doi: 10.1021/ja0352202. J Am Chem Soc. 2003. PMID: 12797773 - Peptide macrocyclization by transition metal catalysis.
Rivera DG, Ojeda-Carralero GM, Reguera L, Van der Eycken EV. Rivera DG, et al. Chem Soc Rev. 2020 Apr 7;49(7):2039-2059. doi: 10.1039/c9cs00366e. Chem Soc Rev. 2020. PMID: 32142086 Review.
Cited by
- Activity and Biocatalytic Potential of an Indolylamide Generating Thioesterase.
Zhong W, Budimir ZL, Johnson LO, Parkinson EI, Agarwal V. Zhong W, et al. Org Lett. 2024 Nov 1;26(43):9378-9382. doi: 10.1021/acs.orglett.4c03648. Epub 2024 Oct 21. Org Lett. 2024. PMID: 39432510 Free PMC article. - Chemoenzymatic tandem cyclization for the facile synthesis of bicyclic peptides.
Kobayashi M, Onozawa N, Matsuda K, Wakimoto T. Kobayashi M, et al. Commun Chem. 2024 Mar 28;7(1):67. doi: 10.1038/s42004-024-01147-w. Commun Chem. 2024. PMID: 38548970 Free PMC article. - Cheminformatics-Guided Cell-Free Exploration of Peptide Natural Products.
Pelton JM, Hochuli JE, Sadecki PW, Katoh T, Suga H, Hicks LM, Muratov EN, Tropsha A, Bowers AA. Pelton JM, et al. J Am Chem Soc. 2024 Mar 27;146(12):8016-8030. doi: 10.1021/jacs.3c11306. Epub 2024 Mar 12. J Am Chem Soc. 2024. PMID: 38470819 Free PMC article. - Proteases Involved in Leader Peptide Removal during RiPP Biosynthesis.
Eslami SM, van der Donk WA. Eslami SM, et al. ACS Bio Med Chem Au. 2023 Dec 13;4(1):20-36. doi: 10.1021/acsbiomedchemau.3c00059. eCollection 2024 Feb 21. ACS Bio Med Chem Au. 2023. PMID: 38404746 Free PMC article. Review. - An Autocatalytic Peptide Cyclase Improves Fidelity and Yield of Circular Peptides In Vivo and In Vitro.
Lacerna N 2nd, Cong Y, Schmidt EW. Lacerna N 2nd, et al. ACS Synth Biol. 2024 Jan 19;13(1):394-401. doi: 10.1021/acssynbio.3c00645. Epub 2024 Jan 9. ACS Synth Biol. 2024. PMID: 38194299 Free PMC article.
References
- Kopp F, Marahiel MA. Nat Prod Rep. 2007;24:735–49. - PubMed
- Burton PS, Conradi RA, Ho NF, Hilgers AR, Borchardt RT. J Pharm Sci. 1996;85:1336–40. - PubMed
- Donia MS, Schmidt EW, Lew M, Hung-Wen L. Comprehensive Natural Products II. Elsevier; Oxford: 2010. pp. 539–558.
- Donia MS, Hathaway BJ, Sudek S, Haygood MG, Rosovitz MJ, Ravel J, Schmidt EW. Nat Chem Biol. 2006;2:729–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM071425/GM/NIGMS NIH HHS/United States
- R01 GM071425-04/GM/NIGMS NIH HHS/United States
- R01 GM071425-05/GM/NIGMS NIH HHS/United States
- GM071425/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources