When neurogenesis encounters aging and disease - PubMed (original) (raw)
Review
When neurogenesis encounters aging and disease
Orly Lazarov et al. Trends Neurosci. 2010 Dec.
Abstract
In this review, we consider the evidence that a reduction in neurogenesis underlies aging-related cognitive deficits and impairments in disorders such as Alzheimer's disease (AD). The molecular and cellular alterations associated with impaired neurogenesis in the aging brain are discussed. Dysfunction of presenilin-1, misprocessing of amyloid precursor protein and toxic effects of hyperphosphorylated tau and β-amyloid probably contribute to impaired neurogenesis in AD. Because factors such as exercise, environmental enrichment and dietary energy restriction enhance neurogenesis, and protect against age-related cognitive decline and AD, knowledge of the underlying neurogenic signaling pathways could lead to novel therapeutic strategies for preserving brain function. In addition, manipulation of endogenous neural stem cells and stem cell transplantation, as stand-alone or adjunct treatments, seems promising.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
Figure 1. Neurogenesis in the adult rodent brain
A sagital section of mouse brain shows the neurogenic microenvironments in the adult brain: the subventricular zone (SVZ) and the subgranular layer (SGL) of the dentate gyrus (DG). Stages of morphological and physiological development of neural stem cells (NSCs) in the SVZ (left) and SGL (right) are illustrated in inserts. Specifically, the SGL contains Type I NSCs, type II and type III cells which can be identified by distinct morphological and molecular markers. Type I have radial processes extending into the inner molecular layer. These cells express nestin, glial fibrillary acidic protein (GFAP), mammalian hairy and Enhancer-of-split homologues (Hes5), brain lipid binding protein (BLBP) and Sex determining region Y-box 1,2 (Sox1 and Sox2). This pool of NSCs stays relatively stable throughout life. Type II [neural progenitor cells (NPCs) or intermediate progenitors (IP)] have only short processes if any, and do not express GFAP. Type II cells may arise from type I cells. Type II cells can be divided to two subpopulations: (i) type IIa, which express Mash1 (Ascl1) as well as retaining expression of NSC markers such as Sox2, and (ii) type IIb cells, which are early-committed neuronal progenitors expressing the transcription factors Prospero homeobox protein 1 (Prox1), Neurogenic differentiation 1 (NeuroD1), as well as Doublecortin (Dcx). They continue to proliferate and may give rise to type III cells, which are migratory neuroblasts that express DCX and polysialated neural cell adhesion molecule (PSA-NCAM)-. After a limited number of cell divisions, type III cells exit the cell cycle and become mature granule neurons. Multiple signals in the local niche determine the fate of NSCs. These signals include: soluble factors (purple ovals) such as brain derived neurotrophic factor (BDNF), cell surface signals (yellow squares) such as Notch1, and extracellular matrix (ECM) factors such as laminin (pink triangles). Endothelial cells (EC) and astrocytes (AS) are thought to be supporting cells in the neurogenic niche, providing signals that are important for maintaining and mobilizing the NSC populations [2, 74]. In the SVZ, type B cells resemble SGL type I cells. They express GFAP, nestin, sox1, sox2, BLBP and the astrocyte-specific L-glutamate L-aspartate transporter (GLAST). They give rise to GFAP-negative transit-amplifying type C cells, which then give rise to type A cells. Type A are PSA-NCAM- and DCX-expressing neuroblasts that migrate radially on “glial tubes” in the rostral migratory stream (RMS) to layers in the olfactory bulb (OB) before terminal differentiation [107]. Abbreviations: cc, corpus collosum; hipp, hippocampus).
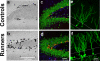
Figure 2. Regulation of cell proliferation and neurogenesis in the hippocampus by exercise
(a,b) Photomicrographs of bromodeoxyuridine (BrdU) positive cells in the dentate gyrus (DG) of adult mice 1 day after the last of a series of 12 daily BrdU injections (50 mg/kg per day). Mice housed with a running wheel (b) have more BrdU+ cells than sedentary mice (a), showing that running increases cell proliferation. (c,d) Confocal images of sections that were immunofluorescent-triple-labeled for BrdU (red), NeuN (green; an indicator of neuronal phenotype), and S100β (blue, -selective for glial phenotype). Neurons double labeled for BrdU+ (red) and NeuN+ (green) appear orange. These images demonstrate that cell survival and neuronal differentiation is enhanced 4 weeks after the last BrdU injection in runners (d) relative to control (c) mice. (e,f) Labeling using a retrovirus (which only infects dividing cells) was used to identify new neurons [111]. Retrovirus expressing green fluorescent protein (GFP) was injected into the DG of sedentary (e) and running (f) mice. Confocal images show more GFP+ new neurons in running mice compared to sedentary mice 4 weeks after virus injection. The dashed lines represent the boundaries of the granule cell layer of the DG. Reproduced, with permission, from Ref. [112].
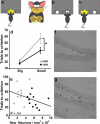
Figure3. Healthy and unhealthy lifestyles may differentially affect hippocampal plasticity and cognitive aging
(a) A working model illustrating mechanisms by which a moderation of dietary energy intake, exercise and a cognitively challenging lifestyle can enhance hippocampal plasticity and sustain cognitive performance into late life. Exercise, dietary energy restriction and intellectual challenges all increase the activation of excitatory input to dendrites of granule neurons (green neurons) in the dentate gyrus. This synaptic activity induces the expression of neurotrophic factors such as brain derived neurotrophic factor (BDNF) and fibroblast growth factor 2 (FGF-2), which have multiple actions on neural stem cells (NSCs) and differentiated neurons that enhance hippocampal functional capability. BDNF signaling increases the strength of potentiated synapses and also acts on NSCs to promote their differentiation into neurons. FGF-2 promotes the proliferation of stem cells to increase the NSC pool available to form new neurons and glial cells. By increasing neuronal network activity, healthy lifestyles can reduce levels of reactive oxygen species (ROS), hence reducing oxidative stress, and bolstering energy metabolism in hippocampal neurons and NSCs, thereby counteracting the aging process. (b) Mechanisms by which excessive energy intake and low levels of energy expenditure adversely affect neurogenesis and cognitive function. Overeating/gluttony, physical inactivity and a cognitively impoverished lifestyle all result in suboptimal levels of input to the hippocampus. The low neuronal network activity, in turn, results in reduced levels of neurotrophic factors such as BDNF and FGF-2, as well as lower levels of neuroprotective protein chaperones and antioxidants. Consequently, NSC proliferation and differentiation into dentate granule neurons is suppressed, and synapses may become dysfunctional and even degenerate. Elevated levels of oxidative and metabolic stress contribute to impaired synaptic plasticity and cognitive impairment. Diagram adapted from Ref. [113].
Figure3. Healthy and unhealthy lifestyles may differentially affect hippocampal plasticity and cognitive aging
(a) A working model illustrating mechanisms by which a moderation of dietary energy intake, exercise and a cognitively challenging lifestyle can enhance hippocampal plasticity and sustain cognitive performance into late life. Exercise, dietary energy restriction and intellectual challenges all increase the activation of excitatory input to dendrites of granule neurons (green neurons) in the dentate gyrus. This synaptic activity induces the expression of neurotrophic factors such as brain derived neurotrophic factor (BDNF) and fibroblast growth factor 2 (FGF-2), which have multiple actions on neural stem cells (NSCs) and differentiated neurons that enhance hippocampal functional capability. BDNF signaling increases the strength of potentiated synapses and also acts on NSCs to promote their differentiation into neurons. FGF-2 promotes the proliferation of stem cells to increase the NSC pool available to form new neurons and glial cells. By increasing neuronal network activity, healthy lifestyles can reduce levels of reactive oxygen species (ROS), hence reducing oxidative stress, and bolstering energy metabolism in hippocampal neurons and NSCs, thereby counteracting the aging process. (b) Mechanisms by which excessive energy intake and low levels of energy expenditure adversely affect neurogenesis and cognitive function. Overeating/gluttony, physical inactivity and a cognitively impoverished lifestyle all result in suboptimal levels of input to the hippocampus. The low neuronal network activity, in turn, results in reduced levels of neurotrophic factors such as BDNF and FGF-2, as well as lower levels of neuroprotective protein chaperones and antioxidants. Consequently, NSC proliferation and differentiation into dentate granule neurons is suppressed, and synapses may become dysfunctional and even degenerate. Elevated levels of oxidative and metabolic stress contribute to impaired synaptic plasticity and cognitive impairment. Diagram adapted from Ref. [113].
Similar articles
- Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice.
Demars M, Hu YS, Gadadhar A, Lazarov O. Demars M, et al. J Neurosci Res. 2010 Aug 1;88(10):2103-17. doi: 10.1002/jnr.22387. J Neurosci Res. 2010. PMID: 20209626 Free PMC article. - Increased hippocampal neurogenesis in the progressive stage of Alzheimer's disease phenotype in an APP/PS1 double transgenic mouse model.
Yu Y, He J, Zhang Y, Luo H, Zhu S, Yang Y, Zhao T, Wu J, Huang Y, Kong J, Tan Q, Li XM. Yu Y, et al. Hippocampus. 2009 Dec;19(12):1247-53. doi: 10.1002/hipo.20587. Hippocampus. 2009. PMID: 19309037 - Deep Brain Magnetic Stimulation Promotes Neurogenesis and Restores Cholinergic Activity in a Transgenic Mouse Model of Alzheimer's Disease.
Zhen J, Qian Y, Fu J, Su R, An H, Wang W, Zheng Y, Wang X. Zhen J, et al. Front Neural Circuits. 2017 Jun 30;11:48. doi: 10.3389/fncir.2017.00048. eCollection 2017. Front Neural Circuits. 2017. PMID: 28713248 Free PMC article. - Adult hippocampal neurogenesis and its role in Alzheimer's disease.
Mu Y, Gage FH. Mu Y, et al. Mol Neurodegener. 2011 Dec 22;6:85. doi: 10.1186/1750-1326-6-85. Mol Neurodegener. 2011. PMID: 22192775 Free PMC article. Review. - Hippocampal neurogenesis: Learning to remember.
Lazarov O, Hollands C. Lazarov O, et al. Prog Neurobiol. 2016 Mar-May;138-140:1-18. doi: 10.1016/j.pneurobio.2015.12.006. Epub 2016 Feb 6. Prog Neurobiol. 2016. PMID: 26855369 Free PMC article. Review.
Cited by
- A comprehensive evaluation on the associations between hearing and vision impairments and risk of all-cause and cause-specific dementia: results from cohort study, meta-analysis and Mendelian randomization study.
Jiang F, Dong Q, Wu S, Liu X, Dayimu A, Liu Y, Ji H, Wang L, Liu T, Li N, Li X, Fu P, Jing Q, Zhou C, Li H, Xu L, Chen S, Wang H. Jiang F, et al. BMC Med. 2024 Nov 7;22(1):518. doi: 10.1186/s12916-024-03748-7. BMC Med. 2024. PMID: 39506811 Free PMC article. - Polyphenol-based polymer nanoparticles for inhibiting amyloid protein aggregation: recent advances and perspectives.
Fang S, Zhang K, Liu D, Yang Y, Xi H, Xie W, Diao K, Rao Z, Wang D, Yang W. Fang S, et al. Front Nutr. 2024 Jul 29;11:1408620. doi: 10.3389/fnut.2024.1408620. eCollection 2024. Front Nutr. 2024. PMID: 39135555 Free PMC article. Review. - Exploring the Role of Neuroplasticity in Development, Aging, and Neurodegeneration.
Marzola P, Melzer T, Pavesi E, Gil-Mohapel J, Brocardo PS. Marzola P, et al. Brain Sci. 2023 Nov 21;13(12):1610. doi: 10.3390/brainsci13121610. Brain Sci. 2023. PMID: 38137058 Free PMC article. Review. - Reduced neurogenesis in human hippocampus with Alzheimer's disease.
Cao Y, Liu P, Bian H, Jin S, Liu J, Yu N, Cui H, Sun F, Qian X, Qiu W, Ma C. Cao Y, et al. Brain Pathol. 2024 May;34(3):e13225. doi: 10.1111/bpa.13225. Epub 2023 Nov 27. Brain Pathol. 2024. PMID: 38012054 Free PMC article. - Dexmedetomidine alleviates cognitive impairment by promoting hippocampal neurogenesis via BDNF/TrkB/CREB signaling pathway in hypoxic-ischemic neonatal rats.
Chen X, Chen A, Wei J, Huang Y, Deng J, Chen P, Yan Y, Lin M, Chen L, Zhang J, Huang Z, Zeng X, Gong C, Zheng X. Chen X, et al. CNS Neurosci Ther. 2024 Jan;30(1):e14486. doi: 10.1111/cns.14486. Epub 2023 Oct 13. CNS Neurosci Ther. 2024. PMID: 37830170 Free PMC article.
References
- Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nature reviews Mol Cell Biol. 2007;8(9):703–713. - PubMed
- Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–275. - PubMed
- Carleton A, et al. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6(5):507–518. - PubMed
- Platel JC, Lacar B, Bordey A. GABA and glutamate signaling: homeostatic control of adult forebrain neurogenesis. J Mol Histol. 2007;38(6):602–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG22555/AG/NIA NIH HHS/United States
- R01 AG033570-01/AG/NIA NIH HHS/United States
- RC1 AG036208/AG/NIA NIH HHS/United States
- RC1 AG036208-01/AG/NIA NIH HHS/United States
- R01 AG026146/AG/NIA NIH HHS/United States
- AG20047/AG/NIA NIH HHS/United States
- AG033570/AG/NIA NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- AG036208Z/AG/NIA NIH HHS/United States
- R01 AG022555/AG/NIA NIH HHS/United States
- R01 AG020047/AG/NIA NIH HHS/United States
- RC1 AG036208-02/AG/NIA NIH HHS/United States
- RF1 AG033570/AG/NIA NIH HHS/United States
- AG026146/AG/NIA NIH HHS/United States
- R01 AG033570/AG/NIA NIH HHS/United States
- R01 AG033570-02/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical