Hypoxia and hypoxia-inducible factors: master regulators of metastasis - PubMed (original) (raw)
Review
Hypoxia and hypoxia-inducible factors: master regulators of metastasis
Xin Lu et al. Clin Cancer Res. 2010.
Abstract
Hypoxia is a common condition found in a wide range of solid tumors and is often associated with poor prognosis. Hypoxia increases tumor glycolysis, angiogenesis, and other survival responses, as well as invasion and metastasis by activating relevant gene expressions through hypoxia-inducible factors (HIF). HIF-1α and HIF-2α undergo oxygen-dependent regulation, and their overexpression is frequently associated with metastasis and poor clinical outcomes. Recent studies show that each step of the metastasis process, from the initial epithelial-mesenchymal transition to the ultimate organotropic colonization, can potentially be regulated by hypoxia, suggesting a master regulator role of hypoxia and HIFs in metastasis. Furthermore, modulation of cancer stem cell self-renewal by HIFs may also contribute to the hypoxia-regulated metastasis program. The hypoxia-induced metastatic phenotype may be one of the reasons for the modest efficacy of antiangiogenic therapies and may well explain the recent provocative findings that antiangiogenic therapy increased metastasis in preclinical models. Multiple approaches to targeting hypoxia and HIFs, including HIF inhibitors, hypoxia-activated bioreductive prodrugs, and gene therapies may become effective treatments to prevent or reduce metastasis.
©2010 AACR.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures
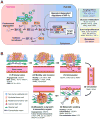
Figure 1. Hypoxia regulation of the metastasis cascade
(A) In the presence of oxygen (O2), PHDs hydroxylate proline residues of HIF-1α, a reaction that can be inhibited by iron chelation. Hydroxylated HIF-1α interacts with VHL, which is part of an E3 ubiquitin ligase complex that mediates the ubiquitination (Ub) of HIF-1α and targets HIF-1α for degradation in the proteasome. Under hypoxia, HIF-1α is not hydroxylated and is released from VHL-mediated degradation. Stabilized HIF-1 moves into the nucleus, dimerizes with HIF-1β and binds to the hypoxia response element (HRE). By interacting with cofactors such as CBP/p300 and DNA polymerase II, HIF-1 activates transcription of target genes. Binding to CBP/p300 is blocked when HIF-1α is hydroxylated by FIH-1. HIF-1α is additionally regulated by oncogenic pathways (e.g., ERBB2, SRC, ET-1, RAS/MARK pathway, PI3K-Akt-mTOR pathway), mutations of tumor suppressor genes (e.g., PTEN, VHL, SDH and FH), and reactive oxygen species (ROS). HIF-1 target genes related to cancer are categorized into four groups, with representative genes in each group listed. (B) The role of hypoxia responsive genes in different steps of metastasis. 1) The primary response to hypoxia during primary tumor growth is the induction of angiogenesis through VEGFA. HIFs also enhance cancer stem cell self-renewal ability partly through DLK1. Hypoxia activates the expression of SNAIL, TWIST1, TCF3, ZEB1 and ZEB2 to promote epithelial-mesenchymal transition of tumor cells. 2) Tumor cells acquire additional motility under hypoxia with increased expression of LOX, MET and AMF. Furthermore, hypoxia promotes invasion through basement membrane (BM) and extracellular matrix (ECM) by upregulating proteases such as CTSD, uPAR and MMPs. Increased production of fibronectin by hypoxia facilitates the establishment of a tumor-specific ECM. 3) Hypoxia facilitates intravasation by increasing the expression of VEGFA, miR-372/373 and MMPs. 4) Circulating tumor cells may acquire the resistance to anoikis by activation of TrkB and repression of integrin α5 (ITGA5), both of which are hypoxia targets. 5) After reaching the secondary vasculature, tumor cells extravasate using hypoxia-induced molecules such as ANGPTL4, MMPs and VEGFA. Hypoxia-dependent induction of CXCR4 and LOX mediates tumor cell homing to secondary organs and formation of pre-metastatic niche, respectively. 6) In the parenchyma of the distant organ, hypoxia response factors such as ANGPTL4, CXCR4, DUSP1, CTGF, OPN, IL6, IL8 and MCP1 facilitate tumor-stromal interactions to foster the formation of overt metastasis in distinct organs.
Similar articles
- Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells.
Mimeault M, Batra SK. Mimeault M, et al. J Cell Mol Med. 2013 Jan;17(1):30-54. doi: 10.1111/jcmm.12004. Epub 2013 Jan 10. J Cell Mol Med. 2013. PMID: 23301832 Free PMC article. - ITGA6 is directly regulated by hypoxia-inducible factors and enriches for cancer stem cell activity and invasion in metastatic breast cancer models.
Brooks DL, Schwab LP, Krutilina R, Parke DN, Sethuraman A, Hoogewijs D, Schörg A, Gotwald L, Fan M, Wenger RH, Seagroves TN. Brooks DL, et al. Mol Cancer. 2016 Mar 22;15:26. doi: 10.1186/s12943-016-0510-x. Mol Cancer. 2016. PMID: 27001172 Free PMC article. - Extracellular ATP promotes breast cancer invasion and epithelial-mesenchymal transition via hypoxia-inducible factor 2α signaling.
Yang H, Geng YH, Wang P, Zhou YT, Yang H, Huo YF, Zhang HQ, Li Y, He HY, Tian XX, Fang WG. Yang H, et al. Cancer Sci. 2019 Aug;110(8):2456-2470. doi: 10.1111/cas.14086. Epub 2019 Jul 10. Cancer Sci. 2019. PMID: 31148343 Free PMC article. - Hypoxia-mediated metastasis.
Chang J, Erler J. Chang J, et al. Adv Exp Med Biol. 2014;772:55-81. doi: 10.1007/978-1-4614-5915-6_3. Adv Exp Med Biol. 2014. PMID: 24272354 Review. - Hypoxia-Inducible Factors: Master Regulators of Cancer Progression.
Schito L, Semenza GL. Schito L, et al. Trends Cancer. 2016 Dec;2(12):758-770. doi: 10.1016/j.trecan.2016.10.016. Epub 2016 Nov 16. Trends Cancer. 2016. PMID: 28741521 Review.
Cited by
- Optical imaging of gastric cancer with near-infrared heptamethine carbocyanine fluorescence dyes.
Zhao N, Zhang C, Zhao Y, Bai B, An J, Zhang H, Wu JB, Shi C. Zhao N, et al. Oncotarget. 2016 Aug 30;7(35):57277-57289. doi: 10.18632/oncotarget.10031. Oncotarget. 2016. PMID: 27329598 Free PMC article. - Exosomes Derived from Hypoxic Colorectal Cancer Cells Transfer miR-410-3p to Regulate Tumor Progression.
Hu X, Mu Y, Liu J, Mu X, Gao F, Chen L, Wu H, Wu H, Liu W, Zhao Y. Hu X, et al. J Cancer. 2020 May 25;11(16):4724-4735. doi: 10.7150/jca.33232. eCollection 2020. J Cancer. 2020. PMID: 32626519 Free PMC article. - Real-time determination of intracellular oxygen in bacteria using a genetically encoded FRET-based biosensor.
Potzkei J, Kunze M, Drepper T, Gensch T, Jaeger KE, Büchs J. Potzkei J, et al. BMC Biol. 2012 Mar 22;10:28. doi: 10.1186/1741-7007-10-28. BMC Biol. 2012. PMID: 22439625 Free PMC article. - Tumor microenvironment heterogeneity an important mediator of prostate cancer progression and therapeutic resistance.
Ge R, Wang Z, Cheng L. Ge R, et al. NPJ Precis Oncol. 2022 May 4;6(1):31. doi: 10.1038/s41698-022-00272-w. NPJ Precis Oncol. 2022. PMID: 35508696 Free PMC article. Review. - The Lymph Node Microenvironment May Invigorate Cancer Cells With Enhanced Metastatic Capacities.
Li T, Liu T, Zhao Z, Xu X, Zhan S, Zhou S, Jiang N, Zhu W, Sun R, Wei F, Feng B, Guo H, Yang R. Li T, et al. Front Oncol. 2022 Feb 28;12:816506. doi: 10.3389/fonc.2022.816506. eCollection 2022. Front Oncol. 2022. PMID: 35295999 Free PMC article.
References
- Harris AL. Hypoxia [mdash] a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–7. - PubMed
- Semenza GL. Targeting HIF-1 for cancer therapy. Nature Rev Cancer. 2003;3:721–2. - PubMed
- Winter SC, Buffa FM, Silva P, et al. Relation of a hypoxia metagene derived from head and neck cancer to prognosis of multiple cancers. Cancer Res. 2007;67:3441–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources