Targeted delivery of siRNA to hepatocytes and hepatic stellate cells by bioconjugation - PubMed (original) (raw)
. 2010 Nov 17;21(11):2119-27.
doi: 10.1021/bc100346n. Epub 2010 Oct 21.
Affiliations
- PMID: 20964335
- PMCID: PMC2998752
- DOI: 10.1021/bc100346n
Targeted delivery of siRNA to hepatocytes and hepatic stellate cells by bioconjugation
Lin Zhu et al. Bioconjug Chem. 2010.
Abstract
Previously, we successfully conjugated galactosylated poly(ethylene glycol) (Gal-PEG) to oligonucleotides (ODNs) via an acid labile ester linker (Zhu et al., Bioconjugate Chem. 2008, 19, 290-8). In this study, sense strands of siRNA were conjugated to Gal-PEG and mannose 6-phosphate poly(ethylene glycol) (M6P-PEG) for targeted delivery of siRNAs to hepatocytes and hepatic stellate cells (HSCs), respectively. These siRNA conjugates were purified by ion exchange chromatography and verified by gel retardation assay. To evaluate their RNAi functions, the validated siRNA duplexes targeting firefly luciferase and transforming growth factor beta 1 (TGF-β1) mRNA were conjugated to Gal-PEG and M6P-PEG, and their gene silencing efficiencies were determined after transfection into HepG2 and HSC-T6 cells. The disulfide bond between PEG and siRNA was cleaved by dithiothreitol, leading to the release of intact siRNA. Both Gal-PEG-siRNA and M6P-PEG-siRNA conjugates could silence luciferase gene expression by about 40% without any transfection reagents, while the gene silencing effects reached more than 98% with the help of cationic liposomes at the same dose. Conjugation of TGF-β1 siRNA with Gal-PEG and M6P-PEG could silence endogenous TGF-β1 gene expression as well. In conclusion, these siRNA conjugates have the potential for targeted delivery of siRNAs to hepatocytes and hepatic stellate cells for efficient gene silencing in vivo.
Figures
Figure 1
Synthesis scheme of Gal-PEG-siRNA and M6P-PEG-siRNA
Figure 2
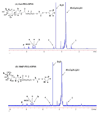
Synthesis and characterization of p-aminophenyl-6-phospho-α-D-mannopyranoside with ESI-MS and 1H NMR. A) Synthesis scheme of p-aminophenyl-6-phospho-α-D-mannopyranoside. B) Electron spray ionization mass spectra were obtained after dissolving the product in a 4:1 (v/v) methanol-water mixture on an ESQUIRE-LC Ion Trap LC/MS system in negative mode. C) For 1H NMR, samples were dissolved in D2O and the spectra were recorded on a Bruker ARX-500 MHz NMR spectrometer at 25 °C.
Figure 3
Characterization of Gal-PEG-OPSS (A) and M6P-PEG-OPSS (B) with 1H NMR. The samples were dissolved in D2O and the spectra were recorded on a Bruker ARX-500 MHz NMR spectrometer at 25 °C.
Figure 4
Purification of M6P-PEG-siRNA conjugate by Ion-Exchange Chromatography. The reaction mixture was carried on a Resource™ Q ion exchange column (GE Healthcare Life Sciences, Piscataway, NJ) by an HPLC system (Waters, Milford, MA) with detection at 260 nm using a gradient starting from 0% B to 80% B (A: 20 mM Tris-HCl buffer, B: 0.1 M sodium chloride buffer) at a flow rate of 1 mL/min at 25 °C.
Figure 5
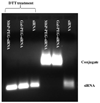
Identification of siRNA conjugates by gel retardation assay. Two micrograms of siRNAs, Gal-PEG-siRNA, and M6P-PEG-siRNA were incubated with 50 mM DTT at the room temperature for 40 min. The treated samples and non-treated controls were applied on 1% agarose gel and run at 10 V/cm for 60 min followed by EtBr staining, then visualized under UV light.
Figure 6
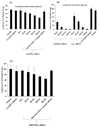
Luciferase gene silencing effects of Gal-PEG-siRNA and M6P-PEG-siRNA. Before gene silencing study, luciferase plasmid was transfected into both HepG2 and HSC-T6 cells using lipoplexes formed between luciferase plasmid and pyridinium cationic liposomes at the charge ratio of 3/1 (+/−). The dose of plasmid was 0.2 µg per well in a 24-well plate. To study the gene silencing ability of Gal-PEG-siRNA, luciferase siRNA and its Gal-PEG-siRNA conjugate were added into HepG2 cells without (A) and with (B) the formation of siRNA/liposomes complexes using pyridinium cationic liposomes. For M6P-PEG-siRNA, luciferas siRNA and its M6P-PEG-siRNA conjugate were added directly into HSC-T6 cells without cationic liposomes (C). After 6h of transfection, the medium was replaced with the complete growth medium and incubated for additional 42h. The cell number was about 4 × 104 cells per well in a 24-well plate.
Figure 7
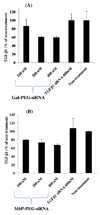
TGF-β1 gene silencing effects of Gal-PEG-siRNA and M6P-PEG-siRNA. A) TGF-β1 siRNA and Gal-PEG-siRNA were added directly into HepG2 cells without cationic liposomes. B) TGF-β1 siRNA and M6P-PEG-siRNA were added directly into HSC-T6 cells without cationic liposomes. After 6h of transfection, the medium was replaced with the complete growth medium and incubated for additional 42h. The cell number was about 4 × 104 cells per well in a 24-well plate.
Similar articles
- Hepatocyte-targeted psiRNA delivery mediated by galactosylated poly(ethylene glycol)-graft-polyethylenimine in vitro.
Nie C, Liu C, Chen G, Dai J, Li H, Shuai X. Nie C, et al. J Biomater Appl. 2011 Sep;26(3):255-75. doi: 10.1177/0885328210364678. Epub 2010 May 28. J Biomater Appl. 2011. PMID: 20511388 - Chemically defined polyethylene glycol siRNA conjugates with enhanced gene silencing effect.
Gaziova Z, Baumann V, Winkler AM, Winkler J. Gaziova Z, et al. Bioorg Med Chem. 2014 Apr 1;22(7):2320-6. doi: 10.1016/j.bmc.2014.02.004. Epub 2014 Feb 20. Bioorg Med Chem. 2014. PMID: 24613624 Free PMC article. - Dicer-labile PEG conjugates for siRNA delivery.
Kow SC, McCarroll J, Valade D, Boyer C, Dwarte T, Davis TP, Kavallaris M, Bulmus V. Kow SC, et al. Biomacromolecules. 2011 Dec 12;12(12):4301-10. doi: 10.1021/bm201199c. Epub 2011 Nov 21. Biomacromolecules. 2011. PMID: 22053777 - Targeted TFO delivery to hepatic stellate cells.
Yang N, Singh S, Mahato RI. Yang N, et al. J Control Release. 2011 Oct 30;155(2):326-30. doi: 10.1016/j.jconrel.2011.06.037. Epub 2011 Jul 8. J Control Release. 2011. PMID: 21763370 Free PMC article. Review. - Structural Modifications of siRNA Improve Its Performance In Vivo.
Chernikov IV, Ponomareva UA, Chernolovskaya EL. Chernikov IV, et al. Int J Mol Sci. 2023 Jan 4;24(2):956. doi: 10.3390/ijms24020956. Int J Mol Sci. 2023. PMID: 36674473 Free PMC article. Review.
Cited by
- Evolution of complexity in non-viral oligonucleotide delivery systems: from gymnotic delivery through bioconjugates to biomimetic nanoparticles.
Bakowski K, Vogel S. Bakowski K, et al. RNA Biol. 2022 Jan;19(1):1256-1275. doi: 10.1080/15476286.2022.2147278. RNA Biol. 2022. PMID: 36411594 Free PMC article. Review. - Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis.
Kumar V, Xin X, Ma J, Tan C, Osna N, Mahato RI. Kumar V, et al. Adv Drug Deliv Rev. 2021 Sep;176:113888. doi: 10.1016/j.addr.2021.113888. Epub 2021 Jul 24. Adv Drug Deliv Rev. 2021. PMID: 34314787 Free PMC article. Review. - Molecular Mechanism for Selective Cytotoxicity towards Cancer Cells of Diselenide-Containing Paclitaxel Nanoparticles.
Li J, Gu Y, Zhang W, Bao CY, Li CR, Zhang JY, Liu T, Li S, Huang JX, Xie ZG, Hua SC, Wan Y. Li J, et al. Int J Biol Sci. 2019 Jul 3;15(8):1755-1770. doi: 10.7150/ijbs.34878. eCollection 2019. Int J Biol Sci. 2019. PMID: 31360117 Free PMC article. - Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery.
Torchilin VP. Torchilin VP. Nat Rev Drug Discov. 2014 Nov;13(11):813-27. doi: 10.1038/nrd4333. Epub 2014 Oct 7. Nat Rev Drug Discov. 2014. PMID: 25287120 Free PMC article. Review. - Glycosylation shapes the efficacy and safety of diverse protein, gene and cell therapies.
Rocamora F, Peralta AG, Shin S, Sorrentino J, Wu MYM, Toth EA, Fuerst TR, Lewis NE. Rocamora F, et al. Biotechnol Adv. 2023 Oct;67:108206. doi: 10.1016/j.biotechadv.2023.108206. Epub 2023 Jun 22. Biotechnol Adv. 2023. PMID: 37354999 Free PMC article. Review.
References
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
- Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347–351. - PubMed
- Oh YK, Park TG. siRNA delivery systems for cancer treatment. Adv Drug Deliv Rev. 2009;61:850–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EB003922/EB/NIBIB NIH HHS/United States
- R01 EB003922-03/EB/NIBIB NIH HHS/United States
- R01 EB003922-04/EB/NIBIB NIH HHS/United States
- EB003922/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources