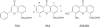Temporal aspects of the action of ASA404 (vadimezan; DMXAA) - PubMed (original) (raw)
Review
Temporal aspects of the action of ASA404 (vadimezan; DMXAA)
Bruce C Baguley et al. Expert Opin Investig Drugs. 2010 Nov.
Abstract
Importance of the field: Tumor vascular disrupting agents (tumor VDAs) act by selective induction of tumor vascular failure. While their action is distinct from that of antiangiogenic agents, their clinical potential is likely to reside in improving the efficacy of combination therapy.
Areas covered in this review: This review describes the preclinical development, clinical trial and mode of action of ASA404, a flavonoid class tumor VDA. This class has a unique dual action, simultaneously disrupting vascular endothelial function and stimulating innate tumor immunity. This review covers the early development of ASA404, through to Phase III trial.
What the reader will gain: The reader will gain insight into the sequence of ASA404-induced changes in tumor tissue. Early events include increased vascular permeability, increased endothelial apoptosis and decreased blood flow, while later effects include the induction of serotonin, tumor necrosis factor, other cytokines and chemokines, and nitric oxide. This cascade of events induces sustained reduction of tumor blood flow, induction of tumor hypoxia and increased inflammatory responses. The reader will also gain an appreciation of how the potentiation of radiation and chemotherapeutic effects by ASA404 in murine tumors shaped the development of combination clinical trials.
Take home message: Although there are species differences in ASA404 activity, many features of its action in mice translate to human studies. The future of ASA404 as an effective clinical agent will rely on the development of an appreciation of its ability to optimize the complex interaction between tumor vasculature and tumor immunity during therapy.
Figures
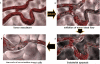
Figure 1
Conceptual illustration of temporal changes in tumor vasculature following administration of a Tumor-VDA.
Figure 2
Theoretical relationship between vascular permeability and blood flow. Tumor vasculature is already partially compromised because of increased vascular permeability and angiogenesis. An anti-angiogenic drug acts to decrease angiogenesis and normalize vascular permeability, while a Tumor-VDA acts by further compromising permeability, leading to further reduction of blood flow and possible catastrophic failure.
Figure 3
Chemical structures of FAA, XAA and ASA404.
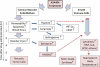
Figure 4
Scheme showing the approximate time course of ASA404 action in mice; the vertical axis indicates the approximate time scale and the shaded boxes represent the effects that contribute to the cascade of vascular disruption. ASA404 facilitates changes in both tumor endothelium and innate immune cells such as macrophages. Its direct effect is limited by a relatively short plasma half-life but leads in vascular endothelial cells to decreased blood flow, increased tumor hypoxia and vascular injury. Early effects on innate immune cells appear to involve increased ceramide production (BC Baguley, unpublished) and later effects involved the production of inflammatory cytokines and nitric oxide. The induction of hypoxia may potentiate responses of innate immune cells and can also induce the cytokine VEGF, which increases vascular permeability. Serotonin produced by platelets in response to vascular injury can potentiate inflammatory cytokine production, as can the appearance of necrotic tumor cells. Co-administration of cytotoxic drugs can also induce tumor cells to induce HMGB1 and other mediators that further stimulate host cytokine responses.
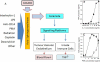
Figure 5
A. A model indicating the possible involvement of the ceramide family of lipid signaling molecules in the action of ASA404. A diverse range of agents is known to simulate the ability of acid sphingomyelinase to synthesize ceramides from sphingomyelin. ASA404 is known to potentiate the ability of several of these agents to stimulate TNF production by cultured human peripheral blood leucocytes (HPBL) (86); two illustrative graphs redrawn from this publication are shown in B and C. Increased concentrations of cellular ceramides facilitate the activation of signaling platforms that can lead to physiological changes in both the tumor vascular endothelium and cells of the innate immune system (see text). B. TNF concentrations of supernatants of HPBL cultures incubated for 8 hours with the indicated concentrations of interleukin-1 alone (∘) or in combination with ASA404 (■). C. TNF concentrations of supernatants of HPBL cultures incubated for 8 hours with the indicated concentrations of phorbol myristate acetate (PMA) alone (∘) or in combination with ASA404 (■). Vertical lines represent the ranges of duplicate cultures.
Similar articles
- The development of the tumor vascular-disrupting agent ASA404 (vadimezan, DMXAA): current status and future opportunities.
Head M, Jameson MB. Head M, et al. Expert Opin Investig Drugs. 2010 Feb;19(2):295-304. doi: 10.1517/13543780903540214. Expert Opin Investig Drugs. 2010. PMID: 20050824 - ASA404: update on drug development.
Rehman F, Rustin G. Rehman F, et al. Expert Opin Investig Drugs. 2008 Oct;17(10):1547-51. doi: 10.1517/13543784.17.10.1547. Expert Opin Investig Drugs. 2008. PMID: 18808313 Review. - An overview on Vadimezan (DMXAA): The vascular disrupting agent.
Daei Farshchi Adli A, Jahanban-Esfahlan R, Seidi K, Samandari-Rad S, Zarghami N. Daei Farshchi Adli A, et al. Chem Biol Drug Des. 2018 May;91(5):996-1006. doi: 10.1111/cbdd.13166. Epub 2018 Jan 24. Chem Biol Drug Des. 2018. PMID: 29288534 Review. - Disrupting established tumor blood vessels: an emerging therapeutic strategy for cancer.
McKeage MJ, Baguley BC. McKeage MJ, et al. Cancer. 2010 Apr 15;116(8):1859-71. doi: 10.1002/cncr.24975. Cancer. 2010. PMID: 20166210 Review. - ASA404: a tumor vascular-disrupting agent with broad potential for cancer therapy.
Baguley BC, McKeage MJ. Baguley BC, et al. Future Oncol. 2010 Oct;6(10):1537-43. doi: 10.2217/fon.10.122. Future Oncol. 2010. PMID: 21062153 Review.
Cited by
- Carboxyxanthones: Bioactive Agents and Molecular Scaffold for Synthesis of Analogues and Derivatives.
Ribeiro J, Veloso C, Fernandes C, Tiritan ME, Pinto MMM. Ribeiro J, et al. Molecules. 2019 Jan 5;24(1):180. doi: 10.3390/molecules24010180. Molecules. 2019. PMID: 30621303 Free PMC article. Review. - Using macrophage activation to augment immunotherapy of established tumours.
Fridlender ZG, Jassar A, Mishalian I, Wang LC, Kapoor V, Cheng G, Sun J, Singhal S, Levy L, Albelda SM. Fridlender ZG, et al. Br J Cancer. 2013 Apr 2;108(6):1288-97. doi: 10.1038/bjc.2013.93. Epub 2013 Mar 12. Br J Cancer. 2013. PMID: 23481183 Free PMC article. - The STING agonist DMXAA triggers a cooperation between T lymphocytes and myeloid cells that leads to tumor regression.
Weiss JM, Guérin MV, Regnier F, Renault G, Galy-Fauroux I, Vimeux L, Feuillet V, Peranzoni E, Thoreau M, Trautmann A, Bercovici N. Weiss JM, et al. Oncoimmunology. 2017 Jul 7;6(10):e1346765. doi: 10.1080/2162402X.2017.1346765. eCollection 2017. Oncoimmunology. 2017. PMID: 29123960 Free PMC article. - STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment.
Chelvanambi M, Fecek RJ, Taylor JL, Storkus WJ. Chelvanambi M, et al. J Immunother Cancer. 2021 Feb;9(2):e001906. doi: 10.1136/jitc-2020-001906. J Immunother Cancer. 2021. PMID: 33526609 Free PMC article.
References
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. - PubMed
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. - PubMed
- Denekamp J. Vascular endothelium as the vulnerable element in tumours. Acta Radiol Oncol. 1984;23:217–25. - PubMed
- *Pioneering work on selective targeting of tumor vasculature
- Zwi LJ, Baguley BC, Gavin JB, Wilson WR. Blood flow failure as a major determinant in the antitumor action of flavone acetic acid. J Natl Cancer Inst. 1989;81:1005–13. - PubMed
- Milosevic MF, Fyles AW, Hill RP. The relationship between elevated interstitial fluid pressure and blood flow in tumors: a bioengineering analysis. Int J Radiat Oncol Biol Phys. 1999;43:1111–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials