Membranes in balance: mechanisms of sphingolipid homeostasis - PubMed (original) (raw)
Review
Membranes in balance: mechanisms of sphingolipid homeostasis
David K Breslow et al. Mol Cell. 2010.
Abstract
Sphingolipids and their metabolites play key cellular roles both as structural components of membranes and as signaling molecules that mediate responses to physiologic cues and stresses. Despite progress during the last two decades in defining the enzymatic machinery responsible for synthesizing and degrading sphingolipids, comparatively little is known about how these enzymes are regulated to ensure sphingolipid homeostasis. Here, we review new insights into how cells sense and control sphingolipid biosynthesis and transport. We also discuss emerging evidence that sphingolipid metabolism is closely coordinated with that of sterols and glycerolipids and with other processes that occur in the secretory pathway. An improved understanding of sphingolipid homeostasis promises to shed light on basic processes in cell biology and disease, including how cells establish and maintain the complex membrane composition and architecture that is a defining feature of eukaryotic cell biology.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
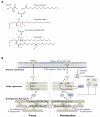
Figure 1. Overview of sphingolipid metabolism in yeast and mammals
(A) Structures of sphingolipid core components are shown, with primary sites of chemical modification and structural diversity highlighted. Long-chain bases (LCBs) are made from the condensation of serine with fatty acid-Coenzyme A (FA-CoA) conjugates. LCBs can be phosphorylated at position a, hydroxylated at position b (yielding phytosphingosine, the major yeast LCB), or desaturated at position c (yielding sphingosine, the major mammalian LCB). Ceramides are produced by N-acylation of LCBs with FA-CoA or very long-chain fatty acid-Coenzyme A (VLCFA-CoA) molecules. The N-linked fatty acids can be hydroxylated at position d and vary in chain length (as indicated by e). The head-group position of ceramides (a) can be phosphorylated as in LCBs. Additionally, the ceramide head-group can be derivatized with inositol and mannose moieties to yield the major yeast sphingolipids, with phosphorylcholine to yield sphingomyelin (SM), or with complex glycans to yield glycosphingolipids (GSLs). (B) Sphingolipid biosynthetic pathways in yeast (left) and mammals (right) are shown, with key steps highlighted. Biosynthesis begins in the ER (bottom) with LCB and ceramide (Cer) production. Ceramides are subsequently transported by vesicular and non-vesicular means to the Golgi, where they undergo modification at the head-group position. In mammalian cells, CERT (Ceramide transport protein) is a ceramide transporter, and FAPP2 (Four-phosphate adaptor protein 2) is a glucosylceramide (GluCer) transporter. In the Golgi, phosphoinositol and mannose moieties are added to yeast ceramides to yield inositolphosphorylceramide (IPC), mannosyl-inositolphosphorylceramide (MIPC), and mannosyl-diinositolphosphorylceramide (M(IP)2C). Similarly, phosphorylcholine and glycan modifications are added to mammalian ceramides in the Golgi to yield SM and GSLs, respectively. Mature sphingolipids are ultimately trafficked to the plasma membrane (top), where they are most abundant. Many of these reactions are reversible, enabling breakdown of complex sphingolipids and generation of signaling molecules such as sphingosine-1-phosphate (S1P). See text for details.
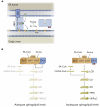
Figure 2. CERT and Orm proteins regulate sphingolipid homeostasis
(A) CERT catalyzes inter-membrane transport of ceramide (Cer; indicated in green) from the ER (top) to the Golgi (bottom). CERT binding to ER membranes is mediated by an FFAT motif that interacts with the ER-localized protein VAP; binding to Golgi membranes is mediated by a PH domain that recognizes phosphatidylinositol-4-phosphate (PI4P; indicated in orange), a phosphoinositide primarily found in the Golgi. CERT’s START domain binds to ceramides and mediates lipid transfer. The activity of CERT is inhibited by phosphorylation, and this phosphorylation is regulated in response to sphingolipid levels. When sphingolipid levels are low, CERT is dephosphorylated and ceramide transport is stimulated (left). When sphingolipid levels are high, CERT is phosphorylated and ceramide transport is reduced (right). (B) Orm proteins are homeostatic regulators of serine palmitoyltransferase, the ER-localized enzyme that catalyzes the condensation of serine with FA-CoAs in the first and rate-limiting step in de novo sphingolipid synthesis. In yeast, Orm1/2 form a complex (termed the SPOTS complex) that includes serine palmitoyltransferase (Lcb1, Lcb2, and the accessory protein Tsc3) and the phosphoinositide phosphatase Sac1. When cellular sphingolipid levels are sufficient for metabolic needs (left), Orm proteins act as inhibitors of serine palmitoyltransferase activity and limit sphingolipid production. However, when sphingolipid levels are inadequate (right), the Orm proteins are inactivated by phosphorylation, enabling compensatory up-regulation of sphingolipid production to meet metabolic demand. This phosphorylation-induced change in Orm protein activity also reduces the ability of Orm proteins and their binding partners to assemble into higher-order multimeric states.
Figure 3. An integrated view of regulatory pathways in sphingolipid metabolism
Several interconnected pathways link sphingolipid metabolism to glycerolipid and sterol production. Shown here are select regulatory mechanisms for mammalian lipid biosynthesis; see text for details. Dotted lines indicate functional relationships that are not yet fully characterized or that may be indirect. Metabolites and pathways for their inter-conversion are shown in black. Proteins and their regulatory activities are shown in blue. Abbreviations are as follows: Ormdl1/2/3 (_ORM1_-like [_S. cerevisiae_] 1/2/3), Sptlc1/2/3 (Serine palmitoyltransferase long-chain base subunit 1/2/3), Sacm1l (Suppressor of actin mutations 1-like), CERT (Ceramide transport protein), OSBP (Oxysterol-binding protein), SREBP (Sterol regulatory element binding protein).
Similar articles
- Sphingolipid homeostasis in the endoplasmic reticulum and beyond.
Breslow DK. Breslow DK. Cold Spring Harb Perspect Biol. 2013 Apr 1;5(4):a013326. doi: 10.1101/cshperspect.a013326. Cold Spring Harb Perspect Biol. 2013. PMID: 23545423 Free PMC article. Review. - Taming the sphinx: Mechanisms of cellular sphingolipid homeostasis.
Olson DK, Fröhlich F, Farese RV Jr, Walther TC. Olson DK, et al. Biochim Biophys Acta. 2016 Aug;1861(8 Pt B):784-792. doi: 10.1016/j.bbalip.2015.12.021. Epub 2015 Dec 30. Biochim Biophys Acta. 2016. PMID: 26747648 Free PMC article. Review. - Sphingolipid homeostasis in the web of metabolic routes.
Aguilera-Romero A, Gehin C, Riezman H. Aguilera-Romero A, et al. Biochim Biophys Acta. 2014 May;1841(5):647-56. doi: 10.1016/j.bbalip.2013.10.014. Epub 2013 Nov 1. Biochim Biophys Acta. 2014. PMID: 24184514 Review. - Distribution and functions of sterols and sphingolipids.
Hannich JT, Umebayashi K, Riezman H. Hannich JT, et al. Cold Spring Harb Perspect Biol. 2011 May 1;3(5):a004762. doi: 10.1101/cshperspect.a004762. Cold Spring Harb Perspect Biol. 2011. PMID: 21454248 Free PMC article. Review. - Sphingolipid topology and the dynamic organization and function of membrane proteins.
van Meer G, Hoetzl S. van Meer G, et al. FEBS Lett. 2010 May 3;584(9):1800-5. doi: 10.1016/j.febslet.2009.10.020. Epub 2009 Oct 20. FEBS Lett. 2010. PMID: 19837070 Review.
Cited by
- Chemical and genetic rescue of in vivo progranulin-deficient lysosomal and autophagic defects.
Doyle JJ, Maios C, Vrancx C, Duhaime S, Chitramuthu B, Bennett HPJ, Bateman A, Parker JA. Doyle JJ, et al. Proc Natl Acad Sci U S A. 2021 Jun 22;118(25):e2022115118. doi: 10.1073/pnas.2022115118. Proc Natl Acad Sci U S A. 2021. PMID: 34140407 Free PMC article. - Signaling and regulatory functions of bioactive sphingolipids as therapeutic targets in multiple sclerosis.
Podbielska M, Krotkiewski H, Hogan EL. Podbielska M, et al. Neurochem Res. 2012 Jun;37(6):1154-69. doi: 10.1007/s11064-012-0728-y. Epub 2012 Mar 27. Neurochem Res. 2012. PMID: 22451227 Review. - Autophagy regulates sphingolipid levels in the liver.
Alexaki A, Gupta SD, Majumder S, Kono M, Tuymetova G, Harmon JM, Dunn TM, Proia RL. Alexaki A, et al. J Lipid Res. 2014 Dec;55(12):2521-31. doi: 10.1194/jlr.M051862. Epub 2014 Oct 20. J Lipid Res. 2014. PMID: 25332431 Free PMC article. - Fatty Acid Synthesis in Glial Cells of the CNS.
Garcia Corrales AV, Haidar M, Bogie JFJ, Hendriks JJA. Garcia Corrales AV, et al. Int J Mol Sci. 2021 Jul 29;22(15):8159. doi: 10.3390/ijms22158159. Int J Mol Sci. 2021. PMID: 34360931 Free PMC article. Review. - Targeting acid sphingomyelinase reduces cardiac ceramide accumulation in the post-ischemic heart.
Klevstig M, Ståhlman M, Lundqvist A, Scharin Täng M, Fogelstrand P, Adiels M, Andersson L, Kolesnick R, Jeppsson A, Borén J, Levin MC. Klevstig M, et al. J Mol Cell Cardiol. 2016 Apr;93:69-72. doi: 10.1016/j.yjmcc.2016.02.019. Epub 2016 Feb 28. J Mol Cell Cardiol. 2016. PMID: 26930027 Free PMC article.
References
- Alvarez-Vasquez F, Sims K, Cowart L, Okamoto Y, Voit E, Hannun Y. Simulation and validation of modelled sphingolipid metabolism in Saccharomyces cerevisiae. Nature. 2005;433:425–430. - PubMed
- Ariga T, Yu RK. Antiglycolipid antibodies in Guillain-Barre syndrome and related diseases: review of clinical features and antibody specificities. J Neurosci Res. 2005;80:1–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- P50 GM073210/GM/NIGMS NIH HHS/United States
- P50 GM073210-08/GM/NIGMS NIH HHS/United States
- P50 GM073210-06/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources