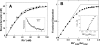Monomeric rhodopsin is sufficient for normal rhodopsin kinase (GRK1) phosphorylation and arrestin-1 binding - PubMed (original) (raw)
Monomeric rhodopsin is sufficient for normal rhodopsin kinase (GRK1) phosphorylation and arrestin-1 binding
Timothy H Bayburt et al. J Biol Chem. 2011.
Abstract
G-protein-coupled receptor (GPCR) oligomerization has been observed in a wide variety of experimental contexts, but the functional significance of this phenomenon at different stages of the life cycle of class A GPCRs remains to be elucidated. Rhodopsin (Rh), a prototypical class A GPCR of visual transduction, is also capable of forming dimers and higher order oligomers. The recent demonstration that Rh monomer is sufficient to activate its cognate G protein, transducin, prompted us to test whether the same monomeric state is sufficient for rhodopsin phosphorylation and arrestin-1 binding. Here we show that monomeric active rhodopsin is phosphorylated by rhodopsin kinase (GRK1) as efficiently as rhodopsin in the native disc membrane. Monomeric phosphorylated light-activated Rh (P-Rh*) in nanodiscs binds arrestin-1 essentially as well as P-Rh* in native disc membranes. We also measured the affinity of arrestin-1 for P-Rh* in nanodiscs using a fluorescence-based assay and found that arrestin-1 interacts with monomeric P-Rh* with low nanomolar affinity and 1:1 stoichiometry, as previously determined in native disc membranes. Thus, similar to transducin activation, rhodopsin phosphorylation by GRK1 and high affinity arrestin-1 binding only requires a rhodopsin monomer.
Figures
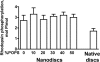
FIGURE 1.
GRK1 efficiently phosphorylates monomeric rhodopsin. Purified rhodopsin from the same batch (50 μg/ml) in original native disc membranes or solubilized and reconstituted into POPC nanodiscs with the indicated fraction of POPS was phosphorylated by purified GRK1 (30 μg/ml) under room light for 10 min at 30 °C. The stoichiometry of phosphorylation was determined, as described under “Experimental Procedures.” Means ± S.D. (error bars) from two experiments performed in duplicate are shown.
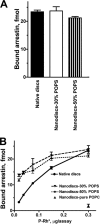
FIGURE 2.
Arrestin-1 binding to monomeric P-Rh*. A, phosphorhodopsin from the same batch (0.3 μg) in native disc membranes or solubilized and reconstituted in POPC nanodiscs with 30 or 50% POPS was incubated with radiolabeled arrestin-1 (100 fmol) in 50 μl at 30 °C. The samples were cooled on ice, and bound and free arrestin-1 was separated, as described under “Experimental Procedures.” B, the same assay was performed with the indicated amounts of P-Rh*. Means ± S.D. from three experiments performed in duplicate are shown.
FIGURE 3.
The dependence of arrestin-1 binding to P-Rh* on negatively charged lipids is reduced by increased ionic strength. A, the binding of radiolabeled arrestin-1 to P-Rh* (0.3 μg) in POPC nanodiscs with the indicated fraction of POPS was performed, as in Fig. 2, at varying total salt concentration (50 m
m
Tris-HCl, pH 7.4, supplemented with 100, 200, 300, or 400 m
m
sodium acetate, pH 7.4). Means ± S.D. of two experiments performed in duplicate are shown. B, to facilitate comparison, means from A are plotted as a percentage of maximum binding at each salt concentration (which is reduced by increasing ionic strength).
FIGURE 4.
Fluorescence-based assay of arrestin-1 binding to monomeric P-Rh* in nanodiscs. A, purified recombinant arrestin-1 with unique cysteine in the C-terminal domain (A348C) was covalently labeled with Texas Red maleimide, and the nanodisc MSP is covalently modified with a quenching group. Free arrestin-1 demonstrates bright fluorescence, whereas the signal from arrestin-1 bound to light-activated P-Rh* in nanodisc is quenched by fluorescence energy transfer. B, change of A348C-Texas Red fluorescence upon photoactivation of P-Rh monomer in nanodiscs. Texas Red fluorescence was monitored at 620 nm. Two-second flashes of light were used to photoactivate rhodopsin (asterisks). The addition of hydroxylamine (indicated by the arrow) released bound arrestin-1 and restored fluorescence.
FIGURE 5.
Affinity and stoichiometry of arrestin-1 interaction with monomeric P-Rh* in nanodiscs. A, arrestin-1 binding isotherms. Measurements were taken at 25 °C with 40 n
m
total arrestin-1 (A348C mutant labeled with Texas Red as in Fig. 4) and 196 n
m
rhodopsin monomer in POPC nanodiscs. Calibrated light exposures were used to titrate in photoactivated rhodopsin. Three separate experiments are shown with fitted KD of 2.5 ± 0.3 n
m
(triangles), 4.2 ± 0.4 n
m
(open circles), or 4.0 ± 0.2 n
m
(circles). The inset shows the fluorescence transient from the first light exposure, fit to a second order reaction. B, titration of arrestin with photoactivated rhodopsin. Measurements were made at a total arrestin concentration of 230 n
m
and P-Rh* monomer nanodiscs at a concentration of 510 n
m
. Photoactivated P-Rh* was titrated in, using calibrated light exposures. KD from fitting of the curve is 16 n
m
. The inset shows transformed data with an x intercept of 1.0, representing the number of arrestin binding sites per P-Rh* monomer.
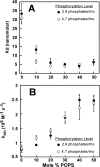
FIGURE 6.
The effect of negatively charged lipids on the affinity of arrestin-1 for P-Rh*. A, dissociation constants obtained from the fluorescence binding assay as a function of POPS content of the nanodiscs. Two preparations of rhodopsin with different phosphorylation levels (as determined by [γ-32P]ATP incorporation) were used in the nanodisc assembly. Binding reactions contained 50 n
m
arrestin and 400 n
m
rhodopsin. B, pseudo-first order kinetic constants were determined from exponential fit to the binding time course. Reactions contained 25 n
m
arrestin. 184 n
m
light-activated rhodopsin in nanodiscs was generated by a 2.5-s light exposure.
FIGURE 7.
Activating mutations differentially change the dependence of arrestin-1 binding to P-Rh* on negatively charged lipids. A, the binding of radiolabeled WT arrestin-1, two polar core mutants (R175E and D296R), and two mutants where the C-tail is either deleted (Tr(1–378)) or detached (arrestin-1 (F375A,V376A,F377A) (3A)) to P-Rh* (0.3 μg) in POPC nanodiscs with the indicated fraction of POPS was performed, as in Fig. 2. Means ± S.D. of two experiments performed in duplicate are shown. B, to facilitate comparison, means are plotted as a percentage of maximum binding of each form of arrestin-1 (which is significantly increased by activating mutations). C, sequence alignment of the C termini of four arrestins with negatively charged residues shown in red. Other highlights are as follows: three bulky hydrophobic residues anchoring the C-tail to the N-domain via β-strand I and α-helix I (olive); arginine that is part of the main phosphate sensor, the polar core (light blue); and the main clathrin-binding site in arrestin-2 (present in all vertebrate non-visual arrestins) (underlined).
Similar articles
- Constitutively active rhodopsin mutants causing night blindness are effectively phosphorylated by GRKs but differ in arrestin-1 binding.
Vishnivetskiy SA, Ostermaier MK, Singhal A, Panneels V, Homan KT, Glukhova A, Sligar SG, Tesmer JJ, Schertler GF, Standfuss J, Gurevich VV. Vishnivetskiy SA, et al. Cell Signal. 2013 Nov;25(11):2155-62. doi: 10.1016/j.cellsig.2013.07.009. Epub 2013 Jul 17. Cell Signal. 2013. PMID: 23872075 Free PMC article. - Evidence that the Rhodopsin Kinase (GRK1) N-Terminus and the Transducin Gα C-Terminus Interact with the Same "Hydrophobic Patch" on Rhodopsin TM5.
Jones Brunette AM, Sinha A, David L, Farrens DL. Jones Brunette AM, et al. Biochemistry. 2016 Jun 7;55(22):3123-35. doi: 10.1021/acs.biochem.6b00328. Epub 2016 May 26. Biochemistry. 2016. PMID: 27078130 - Light-dependent redistribution of visual arrestins and transducin subunits in mice with defective phototransduction.
Zhang H, Huang W, Zhang H, Zhu X, Craft CM, Baehr W, Chen CK. Zhang H, et al. Mol Vis. 2003 Jun 9;9:231-7. Mol Vis. 2003. PMID: 12802257 - The Role of Reversible Phosphorylation of Drosophila Rhodopsin.
Smylla TK, Wagner K, Huber A. Smylla TK, et al. Int J Mol Sci. 2022 Nov 24;23(23):14674. doi: 10.3390/ijms232314674. Int J Mol Sci. 2022. PMID: 36499010 Free PMC article. Review. - Custom-designed proteins as novel therapeutic tools? The case of arrestins.
Gurevich VV, Gurevich EV. Gurevich VV, et al. Expert Rev Mol Med. 2010 Apr 23;12:e13. doi: 10.1017/S1462399410001444. Expert Rev Mol Med. 2010. PMID: 20412604 Free PMC article. Review.
Cited by
- GPCR-dependent and -independent arrestin signaling.
Gurevich VV, Gurevich EV. Gurevich VV, et al. Trends Pharmacol Sci. 2024 Jul;45(7):639-650. doi: 10.1016/j.tips.2024.05.007. Epub 2024 Jun 20. Trends Pharmacol Sci. 2024. PMID: 38906769 Review. - Arrestins: A Small Family of Multi-Functional Proteins.
Gurevich VV. Gurevich VV. Int J Mol Sci. 2024 Jun 6;25(11):6284. doi: 10.3390/ijms25116284. Int J Mol Sci. 2024. PMID: 38892473 Free PMC article. Review. - Nanodiscs for the study of membrane proteins.
Denisov IG, Sligar SG. Denisov IG, et al. Curr Opin Struct Biol. 2024 Aug;87:102844. doi: 10.1016/j.sbi.2024.102844. Epub 2024 May 24. Curr Opin Struct Biol. 2024. PMID: 38795563 Review. - Do arrestin oligomers have specific functions?
Gurevich VV. Gurevich VV. Cell Signal (Middlet). 2023;1(1):42-46. doi: 10.46439/signaling.1.009. Cell Signal (Middlet). 2023. PMID: 37664541 Free PMC article. - Rhodopsins: An Excitingly Versatile Protein Species for Research, Development and Creative Engineering.
de Grip WJ, Ganapathy S. de Grip WJ, et al. Front Chem. 2022 Jun 22;10:879609. doi: 10.3389/fchem.2022.879609. eCollection 2022. Front Chem. 2022. PMID: 35815212 Free PMC article. Review.
References
- Krupnick J. G., Gurevich V. V., Benovic J. L. (1997) J. Biol. Chem. 272, 18125–18131 - PubMed
- Xu J., Dodd R. L., Makino C. L., Simon M. I., Baylor D. A., Chen J. (1997) Nature 389, 505–509 - PubMed
- Fotiadis D., Liang Y., Filipek S., Saperstein D. A., Engel A., Palczewski K. (2003) Nature 421, 127–128 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM081756/GM/NIGMS NIH HHS/United States
- R01 GM077561/GM/NIGMS NIH HHS/United States
- R01 GM081756/GM/NIGMS NIH HHS/United States
- EY011500/EY/NEI NIH HHS/United States
- GM033775/GM/NIGMS NIH HHS/United States
- GM077561/GM/NIGMS NIH HHS/United States
- R01 GM033775/GM/NIGMS NIH HHS/United States
- R01 EY011500/EY/NEI NIH HHS/United States
- R01 HL071818/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources