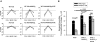Pendrin modulates ENaC function by changing luminal HCO3- - PubMed (original) (raw)
. 2010 Nov;21(11):1928-41.
doi: 10.1681/ASN.2009121257. Epub 2010 Oct 21.
Truyen D Pham, Seongun Hong, Alan M Weinstein, Kathryn B Spencer, Billy Jean Duke, Eric Walp, Young Hee Kim, Roy L Sutliff, Hui-Fang Bao, Douglas C Eaton, Susan M Wall
Affiliations
- PMID: 20966128
- PMCID: PMC3014007
- DOI: 10.1681/ASN.2009121257
Pendrin modulates ENaC function by changing luminal HCO3-
Vladimir Pech et al. J Am Soc Nephrol. 2010 Nov.
Abstract
The epithelial Na(+) channel, ENaC, and the Cl(-)/HCO(3)(-) exchanger, pendrin, mediate NaCl absorption within the cortical collecting duct and the connecting tubule. Although pendrin and ENaC localize to different cell types, ENaC subunit abundance and activity are lower in aldosterone-treated pendrin-null mice relative to wild-type mice. Because pendrin mediates HCO(3)(-) secretion, we asked if increasing distal delivery of HCO(3)(-) through a pendrin-independent mechanism "rescues" ENaC function in pendrin-null mice. We gave aldosterone and NaHCO(3) to increase pendrin-dependent HCO(3)(-) secretion within the connecting tubule and cortical collecting duct, or gave aldosterone and NaHCO(3) plus acetazolamide to increase luminal HCO(3)(-) concentration, [HCO(3)(-)], independent of pendrin. Following treatment with aldosterone and NaHCO(3), pendrin-null mice had lower urinary pH and [HCO(3)(-)] as well as lower renal ENaC abundance and function than wild-type mice. With the addition of acetazolamide, however, acid-base balance as well as ENaC subunit abundance and function was similar in pendrin-null and wild-type mice. We explored whether [HCO(3)(-)] directly alters ENaC abundance and function in cultured mouse principal cells (mpkCCD). Amiloride-sensitive current and ENaC abundance rose with increased [HCO(3)(-)] on the apical or the basolateral side, independent of the substituting anion. However, ENaC was more sensitive to changes in [HCO(3)(-)] on the basolateral side of the monolayer. Moreover, increasing [HCO(3)(-)] on the apical and basolateral side of Xenopus kidney cells increased both ENaC channel density and channel activity. We conclude that pendrin modulates ENaC abundance and function, at least in part by increasing luminal [HCO(3)(-)] and/or pH.
Figures
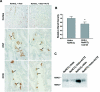
Figure 1.
Acetazolamide reduces pendrin expression in mouse cortex. For 7 days mice were given a NaCl-replete diet with NaHCO3 added to the diet plus an aldosterone infusion (treatment 2). Other mice received this treatment plus acetazolamide (treatment 3). (A) Pendrin labeling in sections of kidney cortex from mice in each group. As shown, pendrin immunolabel was more intense and more discrete in the region of the apical plasma membrane in sections from mice that received aldosterone and NaHCO3 versus sections from mice given NaHCO3, aldosterone, and acetazolamide. (B) Pendrin band density as detected by immunoblot of kidney lysates from mice that received aldosterone and NaHCO3 (n = 12) versus mice that received aldosterone, NaHCO3, and acetazolamide (n = 11). A representative gel is shown on the bottom right panel (C).
Figure 2.
Acetazolamide treatment reduces the predicted differences (between pendrin-null and wild-type mice) in luminal HCO3− concentrations. With use of a mathematical model of aldosterone-stimulated rat distal nephron, concentrations and flows for key solutes were determined in simulations of treatment with aldosterone alone and aldosterone plus NaHCO3 and after administration of aldosterone plus acetazolamide and NaHCO3. Each panel represents the predicted luminal HCO3− concentration as a function of the distance (in cm) downstream of the DCT. Dotted curves were computed using baseline (wild-type) parameters, and solid curves were obtained by setting the density of luminal cell membrane Cl−/HCO3− exchange to 1% of baseline in type B intercalated cells of CNT and CCD (pendrin null). OMCD, outer medullary collecting duct; IMCD, inner medullary collecting duct.
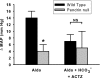
Figure 3.
Acetazolamide treatment restores ENaC activity in CCDs from pendrin-null mice. Panel A shows the effect of benzamil on transepithelial voltage, V
t
, in individual tubules from mice given aldosterone alone, aldosterone plus NaHCO3, or aldosterone plus NaHCO3 plus acetazolamide. Each tubule studied was obtained from a separate mouse. Panel B shows the difference in V
t
measured in the presence (BENZ) and absence (CON-1) of benzamil. Values were compared between pendrin-null and wild-type mice after each treatment protocol using ANOVA.
Figure 4.
Acetazolamide treatment restores ENaC abundance in kidneys from pendrin-null mice. Pendrin-null and wild-type mice were treated as described in Figure 3. α, β, and γ ENaC abundance in whole kidney lysates was quantified by immunoblot from mice in each treatment group studied in parallel. The band density of each lane was normalized to the value obtained from aldosterone-treated wild-type mice run on the same blot. A representative immunoblot is shown (A). (B) α, (C) β, and (D) γ ENaC band density of kidney lysates taken from mice from each treatment group are shown. *P < 0.05, unpaired t test. #P = 0.083. Twelve or 13 animals were studied in each group.
Figure 5.
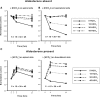
With acetazolamide treatment, aldosterone administration produces a similar change in blood pressure in wild-type and in pendrin-null mice. Changes in mean arterial pressure after treatment with aldosterone alone or aldosterone plus NaHCO3 and acetazolamide were measured in pendrin-null and wild-type mice. After aldosterone treatment, mean arterial BP increased from 116 ± 2 to 130 ± 3 mmHg (n = 9) in wild-type mice, but increased from 111 ± 2 to only 116 ± 2 in pendrin-null mice (n = 8). In contrast, after aldosterone, NaHCO3, and acetazolamide treatment, mean arterial BP increased from 120 ± 3 to 127 ± 3 mmHg (n = 7) in wild-type mice and increased from 112 ± 4 to 117 ± 1 in pendrin-null mice (n = 5). Thus, aldosterone treatment produced a greater rise in BP in wild-type mice relative to pendrin-null mice. However, when NaHCO3 and acetazolamide were added to the treatment protocol, the increment in BP was similar.
Figure 6.
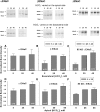
Raising [HCO3−] increases amiloride-sensitive current in mouse principal cells in culture in a time-dependent fashion. mpkCCD cells were cultured in fully defined media for 24 hours before study. HCO3− concentration was then varied on the apical side of the cell by substitution with Cl−, whereas bath HCO3− concentration was kept constant at 30 mM, in the presence (A, n = 3 to 5 for each condition) or absence (C, n = 3 to 5) of aldosterone (10−6 M). Total current was measured at baseline and then after 3, 6, and 24 hours of exposure to media with the indicated HCO3− concentration. Total current at each of these time points was normalized to total current measured at baseline, or at time zero. Total current was lower in cells exposed to 5 mM HCO3− for 24 hours relative to current measured in cells exposed to 15, 30, or 45 mM HCO3− over the same time period either in the presence or in the absence of aldosterone (P < 0.05, ANOVA). However, current did not differ when [HCO3−] on the apical side of the monolayer was 15, 30, or 45 mM. (B and D) Same experiment, but where HCO3− was varied on the basolateral side, while apical HCO3− concentration was kept constant at 30 mM (n = 3 to 6 for each condition). As shown, total current rose in a time-dependent fashion when HCO3− concentration was increased. Statistically significant differences in total current were observed at 24 hours when HCO3− was 5, 15, or 30 mM. However, current was not statistically different when [HCO3−] on the basolateral side was 30 or 45 mM.
Figure 7.
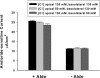
Extracellular Cl− concentration does not modulate amiloride-sensitive current. Cl− concentration, [Cl−], was varied on the apical side (n = 3) or the basolateral side (n = 3) of mpkCCD monolayers over 6 hours, by substituting Cl− for gluconate, while keeping Na+ and HCO3− concentrations constant on both sides of the cell ([Na+] = 160 mM; [HCO3−], 30 mM).
Figure 8.
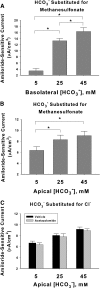
Increasing HCO3− concentration increases amiloride-sensitive current. [HCO3−] was varied on the basolateral side (A, n = 4 for each condition) or on the apical side (B, n = 7 for each condition) of mpkCCD monolayers over 24 hours, by substituting HCO3− for methanesulfonate, whereas Na+ and Cl− concentrations were held constant on both sides of the cell ([Na+] = 165 mM; [Cl−], 120 mM). (C) Effect of varying apical HCO3− concentration by substitution with Cl−. At each HCO3− concentration tested, current was unchanged with acetazolamide (200 μM) present on both sides of the cell. P < 0.05, ANOVA.
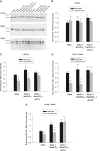
Figure 9.
Raising HCO3− concentration on the apical or the basolateral side of mpkCCD monolayers increases ENaC subunit abundance. α, β, and γ ENaC subunit abundance was quantified by immunoblot when HCO3− concentration was varied on the apical or the basolateral side of mpkCCD monolayers by substituting HCO3− for methanesulfonate in equal concentrations. Band densities are shown in the lower panels. The upper panels display representative gels. *P < 0.05, ANOVA.
Figure 10.
Increasing extracellular HCO3− concentration increases single ENaC channel activity and channel density in A6 cells. (A) Effect of varying HCO3− on ENaC in cell-attached patches in A6 cells treated for 24 hours with 10−6 M aldosterone when HCO3− was varied on the apical and the basolateral side of the cell through substitution with methanesulfonate. (B) Effect on channel density measured as the fraction of patches with measurable channel activity. (C) Channel activity for all patches (NPo) determined by the product of channels times the open probability.
Similar articles
- Pendrin gene ablation alters ENaC subcellular distribution and open probability.
Pech V, Wall SM, Nanami M, Bao HF, Kim YH, Lazo-Fernandez Y, Yue Q, Pham TD, Eaton DC, Verlander JW. Pech V, et al. Am J Physiol Renal Physiol. 2015 Jul 15;309(2):F154-63. doi: 10.1152/ajprenal.00564.2014. Epub 2015 May 13. Am J Physiol Renal Physiol. 2015. PMID: 25972513 Free PMC article. - Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice.
Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin W, Verlander JW, Sutliff RL, Wall SM. Kim YH, et al. Am J Physiol Renal Physiol. 2007 Oct;293(4):F1314-24. doi: 10.1152/ajprenal.00155.2007. Epub 2007 Aug 8. Am J Physiol Renal Physiol. 2007. PMID: 17686956 - Cortical distal nephron Cl(-) transport in volume homeostasis and blood pressure regulation.
Wall SM, Weinstein AM. Wall SM, et al. Am J Physiol Renal Physiol. 2013 Aug 15;305(4):F427-38. doi: 10.1152/ajprenal.00022.2013. Epub 2013 May 1. Am J Physiol Renal Physiol. 2013. PMID: 23637202 Free PMC article. Review. - Deletion of the anion exchanger Slc26a4 (pendrin) decreases apical Cl(-)/HCO3(-) exchanger activity and impairs bicarbonate secretion in kidney collecting duct.
Amlal H, Petrovic S, Xu J, Wang Z, Sun X, Barone S, Soleimani M. Amlal H, et al. Am J Physiol Cell Physiol. 2010 Jul;299(1):C33-41. doi: 10.1152/ajpcell.00033.2010. Epub 2010 Apr 7. Am J Physiol Cell Physiol. 2010. PMID: 20375274 Free PMC article. - The role of pendrin in blood pressure regulation.
Wall SM. Wall SM. Am J Physiol Renal Physiol. 2016 Feb 1;310(3):F193-203. doi: 10.1152/ajprenal.00400.2015. Epub 2015 Nov 4. Am J Physiol Renal Physiol. 2016. PMID: 26538443 Free PMC article. Review.
Cited by
- Pendrin gene ablation alters ENaC subcellular distribution and open probability.
Pech V, Wall SM, Nanami M, Bao HF, Kim YH, Lazo-Fernandez Y, Yue Q, Pham TD, Eaton DC, Verlander JW. Pech V, et al. Am J Physiol Renal Physiol. 2015 Jul 15;309(2):F154-63. doi: 10.1152/ajprenal.00564.2014. Epub 2015 May 13. Am J Physiol Renal Physiol. 2015. PMID: 25972513 Free PMC article. - Pendrin, a novel transcriptional target of the uroguanylin system.
Rozenfeld J, Tal O, Kladnitsky O, Adler L, Efrati E, Carrithers SL, Alper SL, Zelikovic I. Rozenfeld J, et al. Cell Physiol Biochem. 2013;32(7):221-37. doi: 10.1159/000356641. Epub 2013 Dec 18. Cell Physiol Biochem. 2013. PMID: 24429828 Free PMC article. Review. - Epithelial N-methyl-D-aspartate (NMDA) receptors mediate renal vasodilation by affecting kidney autoregulation.
Romero CA, Lim J, Wang H, Wynne BM, Ma P, Jing Y, Liotta DC, D'Erasmo M, Traynelis SF, Eaton DC, Wall SM. Romero CA, et al. bioRxiv [Preprint]. 2023 Dec 6:2023.12.04.569973. doi: 10.1101/2023.12.04.569973. bioRxiv. 2023. PMID: 38106229 Free PMC article. Preprint. - Epithelial anion transporter pendrin contributes to inflammatory lung pathology in mouse models of Bordetella pertussis infection.
Scanlon KM, Gau Y, Zhu J, Skerry C, Wall SM, Soleimani M, Carbonetti NH. Scanlon KM, et al. Infect Immun. 2014 Oct;82(10):4212-21. doi: 10.1128/IAI.02222-14. Epub 2014 Jul 28. Infect Immun. 2014. PMID: 25069981 Free PMC article. - Chemical and Physical Sensors in the Regulation of Renal Function.
Pluznick JL, Caplan MJ. Pluznick JL, et al. Clin J Am Soc Nephrol. 2015 Sep 4;10(9):1626-35. doi: 10.2215/CJN.00730114. Epub 2014 Oct 3. Clin J Am Soc Nephrol. 2015. PMID: 25280495 Free PMC article. Review.
References
- Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang M-E, Everett LA, Green ED, Wall SM: Deoxycorticosterone upregulates Pds (Slc26a4) in mouse kidney: Role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42: 356–362, 2003 - PubMed
- Wall SM, Kim Y-H, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW: NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: Role in Cl- conservation. Hypertension 44: 1–6, 2004 - PubMed
- Verlander JW, Kim Y-H, Shin WK, Pham TD, Hassell KA, Beierwaltes WH, Green ED, Everett LA, Matthews SW, Wall SM: Dietary Cl- restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol 291: F833–F839, 2006 - PubMed
- Kim Y-H, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin WK, Verlander JW, Sutliff RL, Wall SM: Reduced ENaC expression contributes to the lower blood pressure observed in pendrin null mice. Am J Physiol 293: F1314–F1324, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK-P01 06152/DK/NIDDK NIH HHS/United States
- R56 DK052935/DK/NIDDK NIH HHS/United States
- R01 DK052935/DK/NIDDK NIH HHS/United States
- DK-29857/DK/NIDDK NIH HHS/United States
- R01 DK046493/DK/NIDDK NIH HHS/United States
- R01 DK029857/DK/NIDDK NIH HHS/United States
- DK-R37 27963/DK/NIDDK NIH HHS/United States
- DK-52935/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases