miR-29 is a major regulator of genes associated with pulmonary fibrosis - PubMed (original) (raw)
miR-29 is a major regulator of genes associated with pulmonary fibrosis
Leah Cushing et al. Am J Respir Cell Mol Biol. 2011 Aug.
Abstract
MicroRNAs (miRNA) are small regulatory RNAs that control gene expression by translational suppression and destabilization of target mRNAs. There is increasing evidence that miRNAs regulate genes associated with fibrosis in organs, such as the heart, kidney, liver, and the lung. In a large-scale screening for miRNAs potentially involved in bleomycin-induced fibrosis, we found expression of miR-29 family members significantly reduced in fibrotic lungs. Analysis of normal lungs showed the presence of miR-29 in subsets of interstitial cells of the alveolar wall, pleura, and at the entrance of the alveolar duct, known sites of pulmonary fibrosis. miR-29 levels inversely correlated with the expression levels of profibrotic target genes and the severity of the fibrosis. To study the impact of miR-29 down-regulation in the lung interstitium, we characterized gene expression profiles of human fetal lung fibroblast IMR-90 cells in which endogenous miR-29 was knocked down. This confirmed the derepression of reported miR-29 targets, including several collagens, but also revealed up-regulation of a large number of previously unrecognized extracellular matrix-associated and remodeling genes. Moreover, we found that miR-29 is suppressed by transforming growth factor (TGF)-β1 in these cells, and that many fibrosis-associated genes up-regulated by TGF-β1 are derepressed by miR-29 knockdown. Interestingly, a comparison of TGF-β1 and miR-29 targets revealed that miR-29 controls an additional subset of fibrosis-related genes, including laminins and integrins, independent of TGF-β1. Together, these strongly suggest a role of miR-29 in the pathogenesis of pulmonary fibrosis. miR-29 may be a potential new therapeutic target for this disease.
Figures
Figure 1.
Expression of miR-29 in normal and bleomycin-treated mouse lungs. (A) Trichrome staining showed subpleural lesions 7 days after bleomycin injection; progressive fibrosis is shown from Day (D) 7 to D28 with increased collagen (Col) staining; a large portion of these lesions were resolved at D70 and D140. (B) miR-29 family members are down-regulated in bleomycin-treated lungs (*P < 0.05). (C). Levels of miR-29 detected by Northern blot increases during lung development, with highest levels in adult lung. Expression of miR-29 is higher in fibroblast cells (Mlg and NIH3T3) than in epithelial cells (E10, type I–like cells). (D) miR-29 is expressed in the alveolar wall and in pleura. (E1_–_E3) miR-29 is expressed in mesenchymal cells at the entrance of the alveolar duct and costains with α-SMA (B1, miR-29 in situ hybridization [ISH]; B2, α-SMA immunohistochemistry; B3, merged image). Green arrow, subpleural fibrotic lesion; green arrowhead, miR-29–expressing cells; red arrowhead, cells with low or undetectable miR-29 expression.
Figure 2.
Levels of miR-29 inversely correlated with its downstream targets in bleomycin-induced pulmonary fibrosis. (A) Levels of miR-29 are gradually reduced after bleomycin injection, reaching lowest levels at D28, and then gradually recover at D70 and D140. Levels of Col3A1 and Col4A1, targets of miR-29, are inversely correlated with miR-29 levels in this process. (B and C) Down-regulation of miR-29 in cells at the entrance of alveolar ducts is found in bleomycin-treated lungs (C) as compared with controls (B), inversely correlating with collagen staining in these cells (D and E). (B and C) ISH of miR-29c; (D and E) Masson's trichrome staining; *P < 0.05.
Figure 3.
Reduced expression of miR-29 in subepithelial mesenchymal cells of terminal bronchioles was observed in bleomycin-treated lungs (F) as compared with lungs from mice receiving PBS injection (C). This inversely correlates with collagen staining (B and E) and thickening of subepithelial structures (A and D).
Figure 4.
miR-29–mediated regulation of target gene expression. (A) Knockdown of miR-29 expression by locked nucleotide acid (LNA) knockdown oligos. Expression of miR-29a, miR-29b, and miR-29c were significantly reduced in IMR-90 cells (human fetal lung fibroblasts) transfected with LNA knockdown oligos either for miR-29a or miR-29b or miR-29c, but not with scrambled LNA oligos. (B) mRNA levels of COL4A1 and Nidgen (NID) 1 are significantly decreased in IMR-90 cells transiently transfected with miR-29 mimic as compared with control oligos. (C) miR-29 knockdown resulted in a significantly increased level of COL1A1 protein, whereas increased miR-29 reduced COL1A1 protein. (D) The level of total soluble collagen in the culture medium is increased in miR-29 knockdown IMR-90 cells.
Figure 5.
ITGA11, ADAMTS9, ADAM12, and NID1 are direct targets of miR-29. FG293 cells were cotransfected with individual 3′ untranslated region (UTR) luciferase reporters with wild-type (wt) or mutated (mut) miR-29–binding sites, along with miRNA mimics of miR-29 or miR-365. Firefly luciferase activity from the same construct was used to normalize and to generate relative activity of Renilla luciferase, which was subject to the regulation of cloned 3′ UTR. miR-29 significantly suppressed luciferase activities of reporters harboring wild-type 3′ UTR sequences, but not for reporters with mutated miR-29–binding sites. miR-365, an unrelated miRNA, has no significant effect on the luciferase activities of reporters with either wild-type or mutant 3′ UTRs.
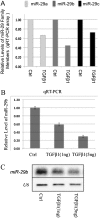
Figure 6.
Expression of miR-29 is down-regulated by transforming growth factor (TGF)–β1 in IMR-90 cells. Down-regulation of miR-29a, -b, and -c were detected by qRT-PCR array (A), and confirmed by quantitative real-time PCR and Northern blot (B and C).
Similar articles
- The anti-fibrotic effects of microRNA-153 by targeting TGFBR-2 in pulmonary fibrosis.
Liang C, Li X, Zhang L, Cui D, Quan X, Yang W. Liang C, et al. Exp Mol Pathol. 2015 Oct;99(2):279-85. doi: 10.1016/j.yexmp.2015.07.011. Epub 2015 Jul 26. Exp Mol Pathol. 2015. PMID: 26216407 - MicroRNA-326 regulates profibrotic functions of transforming growth factor-β in pulmonary fibrosis.
Das S, Kumar M, Negi V, Pattnaik B, Prakash YS, Agrawal A, Ghosh B. Das S, et al. Am J Respir Cell Mol Biol. 2014 May;50(5):882-92. doi: 10.1165/rcmb.2013-0195OC. Am J Respir Cell Mol Biol. 2014. PMID: 24279830 Free PMC article. - Inhibition of lncRNA PFRL prevents pulmonary fibrosis by disrupting the miR-26a/smad2 loop.
Jiang H, Chen Y, Yu T, Zhao X, Shan H, Sun J, Zhang L, Li X, Shan H, Liang H. Jiang H, et al. Am J Physiol Lung Cell Mol Physiol. 2018 Oct 1;315(4):L563-L575. doi: 10.1152/ajplung.00434.2017. Epub 2018 Jun 28. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29952219 - MicroRNAs in idiopathic pulmonary fibrosis.
Pandit KV, Milosevic J, Kaminski N. Pandit KV, et al. Transl Res. 2011 Apr;157(4):191-9. doi: 10.1016/j.trsl.2011.01.012. Epub 2011 Feb 4. Transl Res. 2011. PMID: 21420029 Review. - Role of miRNA and lncRNAs in organ fibrosis and aging.
Ghafouri-Fard S, Abak A, Talebi SF, Shoorei H, Branicki W, Taheri M, Akbari Dilmaghani N. Ghafouri-Fard S, et al. Biomed Pharmacother. 2021 Nov;143:112132. doi: 10.1016/j.biopha.2021.112132. Epub 2021 Sep 1. Biomed Pharmacother. 2021. PMID: 34481379 Review.
Cited by
- Rationale for the Use of Pirfenidone in Heart Failure With Preserved Ejection Fraction.
Graziani F, Lillo R, Crea F. Graziani F, et al. Front Cardiovasc Med. 2021 Apr 22;8:678530. doi: 10.3389/fcvm.2021.678530. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33969025 Free PMC article. Review. - Pathogenesis of Systemic Sclerosis.
Pattanaik D, Brown M, Postlethwaite BC, Postlethwaite AE. Pattanaik D, et al. Front Immunol. 2015 Jun 8;6:272. doi: 10.3389/fimmu.2015.00272. eCollection 2015. Front Immunol. 2015. PMID: 26106387 Free PMC article. Review. - Mechanosensing by the α6-integrin confers an invasive fibroblast phenotype and mediates lung fibrosis.
Chen H, Qu J, Huang X, Kurundkar A, Zhu L, Yang N, Venado A, Ding Q, Liu G, Antony VB, Thannickal VJ, Zhou Y. Chen H, et al. Nat Commun. 2016 Aug 18;7:12564. doi: 10.1038/ncomms12564. Nat Commun. 2016. PMID: 27535718 Free PMC article. - miR-34a Inhibits Lung Fibrosis by Inducing Lung Fibroblast Senescence.
Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Antony VB, Thannickal VJ, Liu G. Cui H, et al. Am J Respir Cell Mol Biol. 2017 Feb;56(2):168-178. doi: 10.1165/rcmb.2016-0163OC. Am J Respir Cell Mol Biol. 2017. PMID: 27635790 Free PMC article. - A miRNA-regulatory network explains how dysregulated miRNAs perturb oncogenic processes across diverse cancers.
Plaisier CL, Pan M, Baliga NS. Plaisier CL, et al. Genome Res. 2012 Nov;22(11):2302-14. doi: 10.1101/gr.133991.111. Epub 2012 Jun 28. Genome Res. 2012. PMID: 22745231 Free PMC article.
References
- Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J 2007;30:835–839. - PubMed
- Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2008;294:L152–L160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases