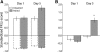Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning - PubMed (original) (raw)
Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning
Mitchell F Roitman et al. Learn Mem. 2010.
Abstract
The nucleus accumbens (NAc) plays a role in hedonic reactivity to taste stimuli. Learning can alter the hedonic valence of a given stimulus, and it remains unclear how the NAc encodes this shift. The present study examined whether the population response of NAc neurons to a taste stimulus is plastic using a conditioned taste aversion (CTA) paradigm. Electrophysiological and electromyographic (EMG) responses to intraoral infusions of a sucrose (0.3 M) solution were made in naïve rats (Day 1). Immediately following the session, half of the rats (n = 6; Paired) received an injection of lithium chloride (0.15 M; i.p.) to induce malaise and establish a CTA while the other half (n = 6; Unpaired) received a saline injection. Days later (Day 5), NAc recordings during infusions of sucrose were again made. Electrophysiological and EMG responses to sucrose did not differ between groups on Day 1. For both groups, the majority of sucrose responsive neurons exhibited a decrease in firing rate (77% and 71% for Paired and Unpaired, respectively). Following conditioning, in Paired rats, EMG responses were indicative of aversion. Moreover, the majority of responsive NAc neurons now exhibited an increase in firing rate (69%). Responses in Unpaired rats were unchanged by the experience. Thus, the NAc differentially encodes the hedonic value of the same stimulus based on learned associations.
Figures
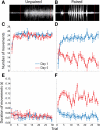
Figure 1.
EMG responses of the anterior digastric muscle to intraorally infused sucrose reflect the formation of a CTA. (A) Representative raw EMG trace recorded from the anterior digastric muscle in an Unpaired rat. (B) Representative raw EMG trace recorded from the anterior digastric muscle in a Paired rat. In both A and B, vertical red lines denote, from left to right, the beginning and end of the 4-sec intraoral infusion of 0.3 M sucrose. In Unpaired rats (n = 6), experience with sucrose (Day 5, red) and LiCl injections did not alter the number (C) or duration (E) of EMG responses across the 30-trial intraoral infusion session relative to the EMG responses observed in the same rats on Day 1 (blue). In Paired rats (n = 6), experience with sucrose (Day 5, red) and LiCl injections led to significant decreases in the number (D) and increases in the duration (F) of EMG responses across the 30-trial intraoral infusion session relative to the EMG responses observed in the same rats on Day 1 (blue).
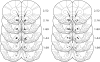
Figure 2.
Histological verification of electrode placements. Lesion sites in Unpaired (left) and Paired (right) rats of confirmed electrode placements in the nucleus accumbens are shown. Placements in the NAc are based on a stereotaxic atlas (Paxinos and Watson 2007).
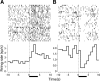
Figure 3.
Raster plots (above) and perievent histograms (below) of two representative cells in the NAc that respond to intraoral infusions of sucrose. (A) An increasing neuron recorded in a Paired rat on Day 5. (B) A decreasing neuron recorded in an Unpaired rat on Day 5. For both graphs, data are aligned to the onset of the infusion (t = 0 sec) and the horizontal black bar denotes the duration of the intraoral infusion.
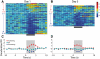
Figure 4.
In Unpaired rats, experience does not affect the population response of NAc neurons to intraoral infusions of sucrose. (Left) Representation of all sucrose responsive NAc neurons recorded on Day 1, under naïve conditions. (A) The colorplot shows the normalized firing rate (1-sec bins) of each neuron that had a statistically significant change in firing rate in response to intraoral infusions. Each row represents the activity of one neuron. Data are aligned to the start of the intraoral infusion (t = 0 sec). Normalized firing rate is shown in color. Neurons are organized such that the strongest increasing neuron is at the top and the strongest decreasing neuron is on the bottom. Increasing neurons are highlighted by the red letter “I” and number. The horizontal dashed white line separates neurons that exhibited a significant increase (above; n = 15) from those that exhibited a significant decrease (n = 36) in firing rate in response to sucrose infusions. Decreasing neurons are highlighted by the blue letter “D” and number. (C) Average normalized response of decreasing (blue circles) and increasing (red circles) neurons as well as the overall population response (green circles) to sucrose infusions on Day 1. (Right) Representation of all sucrose responsive NAc neurons recorded on Day 5, after explicitly Unpaired LiCl experience. (B) Increasing (n = 12) and decreasing (n = 24) neurons recorded on Day 5 are shown as in A. (D) Average normalized response of decreasing (blue circles) and increasing (red circles) neurons as well as the overall population response (green circles) to sucrose infusions on Day 5. The ratio of increasing to decreasing neurons was no different on Day 1 vs. Day 5 (χ2 = 0.00; P > 0.05).
Figure 5.
In Paired rats, experience alters the population response of NAc neurons to intraoral infusions of sucrose. Left: Representation of all sucrose responsive NAc neurons recorded on Day 1, under naïve conditions. (A) The colorplot shows the normalized firing rate (1-sec bins) of each neuron that had a statistically significant change in firing rate in response to intraoral infusions. Each row represents the activity of one neuron. Data are aligned to the start of the intraoral infusion (t = 0 sec). Normalized firing rate is shown in color. Neurons are organized such that the strongest increasing neuron is at the top and the strongest decreasing neuron is on the bottom. Increasing neurons are highlighted by the red letter “I” and number. The horizontal dashed white line separates neurons that exhibited a significant increase (above; n = 8) from those that exhibited a significant decrease (n = 27) in firing rate in response to sucrose infusions. Decreasing neurons are highlighted by the blue letter “D” and number. (C) Average normalized response of decreasing (blue circles) and increasing (red circles) neurons as well as the overall population response (green circles) to sucrose infusions on Day 1. Right: Representation of all sucrose responsive NAc neurons recorded on Day 5, after explicitly Paired LiCl experience. (B) Increasing (n = 27) and decreasing (n = 12) neurons are shown as in A. (D) Average normalized response of decreasing (blue circles) and increasing (red circles) neurons as well as the overall population response (green circles) to sucrose infusions on Day 5. Importantly, the ratio of increasing to decreasing neurons was significantly different on Day 5 vs. Day 1 (χ2 = 47.75; P < 0.001).
Figure 6.
Although the magnitude of increased and decreased responses do not differ across days of testing, the overall response across the population reflects a hedonic shift. (A) The magnitude of the response to sucrose of increasing (solid outlined bars) and decreasing (dashed outlined bars) neurons does not differ based on day (Day 1 vs. Day 5) or pairing (Unpaired vs. Paired). (B) The average firing rate for all sucrose-responsive neurons changes based on pairing. The average firing rate was significantly higher on Day 5 but only in Paired rats. Bars represent means ± 1 SEM. (*) Denotes significant difference (P < 0.001) relative to all other bars.
Similar articles
- Dopamine activity encodes the changing valence of the same stimulus in conditioned taste aversion paradigms.
Loh MK, Hurh SJ, Bazzino P, Donka RM, Keinath AT, Roitman JD, Roitman MF. Loh MK, et al. Elife. 2025 Mar 5;13:RP103260. doi: 10.7554/eLife.103260. Elife. 2025. PMID: 40042246 Free PMC article. - Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: a FOS and behavioral analysis.
Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Kerfoot EC, et al. Learn Mem. 2007 Aug 29;14(9):581-9. doi: 10.1101/lm.627007. Print 2007 Sep. Learn Mem. 2007. PMID: 17761543 Free PMC article. - Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output.
Roitman MF, Wheeler RA, Carelli RM. Roitman MF, et al. Neuron. 2005 Feb 17;45(4):587-97. doi: 10.1016/j.neuron.2004.12.055. Neuron. 2005. PMID: 15721244 - Activation of projective neurons from the nucleus accumbens to ventral pallidum by a learned aversive taste stimulus in rats: a manganese-enhanced magnetic resonance imaging study.
Inui T, Inui-Yamamoto C, Yoshioka Y, Ohzawa I, Shimura T. Inui T, et al. Neuroscience. 2011 Mar 17;177:66-73. doi: 10.1016/j.neuroscience.2011.01.006. Epub 2011 Jan 8. Neuroscience. 2011. PMID: 21219975 - When a good taste turns bad: Neural mechanisms underlying the emergence of negative affect and associated natural reward devaluation by cocaine.
Carelli RM, West EA. Carelli RM, et al. Neuropharmacology. 2014 Jan;76 Pt B(0 0):360-9. doi: 10.1016/j.neuropharm.2013.04.025. Epub 2013 Apr 29. Neuropharmacology. 2014. PMID: 23639430 Free PMC article. Review.
Cited by
- Prefrontal Cortical Opioids and Dysregulated Motivation: A Network Hypothesis.
Baldo BA. Baldo BA. Trends Neurosci. 2016 Jun;39(6):366-377. doi: 10.1016/j.tins.2016.03.004. Trends Neurosci. 2016. PMID: 27233653 Free PMC article. Review. - Ventral arkypallidal neurons inhibit accumbal firing to promote reward consumption.
Vachez YM, Tooley JR, Abiraman K, Matikainen-Ankney B, Casey E, Earnest T, Ramos LM, Silberberg H, Godynyuk E, Uddin O, Marconi L, Le Pichon CE, Creed MC. Vachez YM, et al. Nat Neurosci. 2021 Mar;24(3):379-390. doi: 10.1038/s41593-020-00772-7. Epub 2021 Jan 25. Nat Neurosci. 2021. PMID: 33495635 Free PMC article. - Activation of Infralimbic to Nucleus Accumbens Shell Pathway Suppresses Conditioned Aversion in Male But Not Female Rats.
Hurley SW, Carelli RM. Hurley SW, et al. J Neurosci. 2020 Sep 2;40(36):6888-6895. doi: 10.1523/JNEUROSCI.0137-20.2020. Epub 2020 Jul 29. J Neurosci. 2020. PMID: 32727819 Free PMC article. - Homeostatic regulation of reward via synaptic insertion of calcium-permeable AMPA receptors in nucleus accumbens.
Carr KD. Carr KD. Physiol Behav. 2020 May 15;219:112850. doi: 10.1016/j.physbeh.2020.112850. Epub 2020 Feb 21. Physiol Behav. 2020. PMID: 32092445 Free PMC article. Review. - Coordinated Ramping of Dorsal Striatal Pathways preceding Food Approach and Consumption.
London TD, Licholai JA, Szczot I, Ali MA, LeBlanc KH, Fobbs WC, Kravitz AV. London TD, et al. J Neurosci. 2018 Apr 4;38(14):3547-3558. doi: 10.1523/JNEUROSCI.2693-17.2018. Epub 2018 Mar 9. J Neurosci. 2018. PMID: 29523623 Free PMC article.
References
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D 2001. Reward circuitry activation by noxious thermal stimuli. Neuron 32: 927–946 - PubMed
- Bernstein IL, Chavez M, Allen D, Taylor EM 1992. Area postrema mediation of physiological and behavioral effects of lithium chloride in the rat. Brain Res 575: 132–137 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA025634/DA/NIDA NIH HHS/United States
- DA025679/DA/NIDA NIH HHS/United States
- R01 DA014339/DA/NIDA NIH HHS/United States
- DA014339/DA/NIDA NIH HHS/United States
- R00 DA025679/DA/NIDA NIH HHS/United States
- R21 DA027127/DA/NIDA NIH HHS/United States
- DA027127/DA/NIDA NIH HHS/United States
- DA025634/DA/NIDA NIH HHS/United States
- K99 DA025679/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources