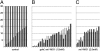Glial cell line-derived neurotrophic factor defines the path of developing and regenerating axons in the lateral line system of zebrafish - PubMed (original) (raw)
Glial cell line-derived neurotrophic factor defines the path of developing and regenerating axons in the lateral line system of zebrafish
Kevin Schuster et al. Proc Natl Acad Sci U S A. 2010.
Abstract
How the peripheral axons of sensory neurons are guided to distant target organs is not well understood. Here we examine this question in the case of the posterior lateral line (PLL) system of zebrafish, where sensory organs are deposited by a migrating primordium. Sensory neurites accompany this primordium during its migration and are thereby guided to their prospective target organs. We show that the inactivation of glial cell line-derived neurotrophic factor (GDNF) signaling leads to defects of innervation and that these defects are due to the inability of sensory axons to track the migrating primordium. GDNF signaling is also used as a guidance cue during axonal regeneration following nerve cut. We conclude that GDNF is a major determinant of directed neuritic growth and of target finding in this system, and we propose that GDNF acts by promoting local neurite outgrowth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
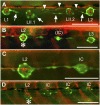
Innervation defects in Huc:kaede, Et20:gfp early larvae. (A) Normal pattern in a control larva at 4 dpf. Nerve branches extend to and arborize in each neuromast. Interneuromast cells deposited by the primordium between L1 and L2 (arrows) have moved ventrally ahead of the primII-derived neuromasts, LII.1 and LII.2. Interneuromast cells deposited by primII can also be observed close to the myoseptum (arrowheads). (B) Peripheral afferent axons stop at L2 (asterisk) in a 3-dpf gdnf, _ret1_-MO1 fish. An intercalary neuromast (IC) is beginning to develop between L2 and L3. The red fibers posterior to L2 belong to motor axons innervating the somitic muscles. (C) A higher magnification photograph to show that no nerve branch extends beyond L2, in a 4-dpf double morphant fish. (D) Nerve stop at L2 (asterisk) in a 4-dpf _gdnf_-MO2 fish. Intercalary neuromasts have formed on all somitic borders posterior to the nerve arrest. Anterior is Left in all panels. (Scale bar, 100 mm.)
Fig. 2.
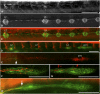
Innervation defects are due to precocious nerve arrest. (A–C) Nerve interruption at L3, in 4-dpf _gdnf, ret1_-MO1 double morphant fish. Nerve arrest (A) is associated with the formation of precocious intercalary neuromasts distal to the interruption (B and C). (D and E) Nerve interruption in double morphant embryos is manifest at a time when the primordium is still migrating in photoconverted Huc:kaede, cldnb:gfp (D) or in Huc:kaede, cxcr4b:rfp (E, arrowhead marks the nerve ending) embryos. (F and G) Arborization of sensory neurites accompanying the migrating primordium at 35 hpf in a control (F) and a _gdnf, ret1_-MO1 double morphant embryo (G). (H) Nerve lagging behind the primordium (arrow) in an nbt:dsred, cldnb:gfp embryo injected with 0.15 mM _ret1_-MO2. L1 is about to be deposited. E and H are composite pictures assembled from two consecutive planes in Z-stacks. Anterior is left in all panels, and the primordium is migrating to the right in D_–_H. (Scale bar, 100 mm.)
Fig. 3.
Partial inactivation of ret1 induces nerve stops, but no neuronal loss, in nbt:dsred, Et20:gfp embryos. The plot represents number of neurons (ordinate) vs. the position reached by the nerve (abscissa) in 19 _ret1_-MO1 embryos raised at 25 °C (squares). Only one side was examined for each embryo. In control embryos (dots) the nerve always reached the terminal neuromasts. (Scale bar, 100 mm.)
Fig. 4.
Still panels from
Movie S3
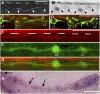
. The nerve was cut just posterior to the ganglion at time 0 in a 3-dpf, nbt:dsred, foxd3:gfp embryo. (A_–_C) Progressive axonal degeneration; (C_–_F) progress of the regenerating growth cones (white arrows). (Scale bar, 100 mm.)
Fig. 5.
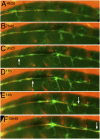
Regenerating axons can be guided by interneuromast cells. (A) After ablation of glial cells in the foxd3:gfp line, new glial cells extend posteriorly (arrowheads). (B) Their path resembles that of interneuromast cells (arrowheads) as visualized by alkaline phosphatase labeling. (C and D) After glial ablation between L1 and L2 in 3-dpf Huc:kaede, Et20:gfp fish, axons regrow along the myoseptum up to LII.1 (C) and follow the interneuromast cells (arrows) up to L2 (arrowhead in D). (E) Regenerating axons in a 54-hpf Huc:kaede, cldnb:gfp fish where the nerve had been cut at 30 hpf. White bars indicate the position of the horizontal myoseptum. (F) Trail of interneuromast cells in the same embryo; (G) merge. (H) Expression of gdnf in the primordium (dotted outline) and in the interneuromast cells (arrows) as revealed by in situ hybridization. (Scale bar, 100 mm.) A and H were assembled from two consecutive levels in Z-stacks.
Fig. 6.
Position reached by the axons at the time the nerve was cut posterior to the ganglion (gray bar) and 3 d later (black bar) in control (A), 1.5 mM _gdnf, ret_-MO1 double morphant (B), and 2.5-mM _ret1_-MO1 embryos (C). (Scale bar, 100 mm.)
Similar articles
- Glial Cell Line-Derived Neurotrophic Factor and Chondroitinase Promote Axonal Regeneration in a Chronic Denervation Animal Model.
Sarhane KA, Tuffaha SH, Ibrahim Z, Cashman CR, Krick K, Martin R, Broyles JM, Cooney DS, Lee WPA, Mi R, Mao HQ, Höke A, Brandacher G. Sarhane KA, et al. Neurotherapeutics. 2019 Oct;16(4):1283-1295. doi: 10.1007/s13311-019-00745-0. Neurotherapeutics. 2019. PMID: 31148054 Free PMC article. - Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury.
Iannotti C, Li H, Yan P, Lu X, Wirthlin L, Xu XM. Iannotti C, et al. Exp Neurol. 2003 Oct;183(2):379-93. doi: 10.1016/s0014-4886(03)00188-2. Exp Neurol. 2003. PMID: 14552879 - Efferent axons in the zebrafish lateral line degenerate following sensory hair cell ablation.
Tuz-Sasik MU, Manuel R, Boije H. Tuz-Sasik MU, et al. Mol Cell Neurosci. 2023 Dec;127:103900. doi: 10.1016/j.mcn.2023.103900. Epub 2023 Sep 13. Mol Cell Neurosci. 2023. PMID: 37714280 - The role of neurotrophic factors in nerve regeneration.
Gordon T. Gordon T. Neurosurg Focus. 2009 Feb;26(2):E3. doi: 10.3171/FOC.2009.26.2.E3. Neurosurg Focus. 2009. PMID: 19228105 Review. - Building the posterior lateral line system in zebrafish.
Chitnis AB, Nogare DD, Matsuda M. Chitnis AB, et al. Dev Neurobiol. 2012 Mar;72(3):234-55. doi: 10.1002/dneu.20962. Dev Neurobiol. 2012. PMID: 21818862 Free PMC article. Review.
Cited by
- Semaphorin7A patterns neural circuitry in the lateral line of the zebrafish.
Dasgupta A, Reagor CC, Paik SP, Snow LM, Jacobo A, Hudspeth AJ. Dasgupta A, et al. Elife. 2024 Aug 12;12:RP89926. doi: 10.7554/eLife.89926. Elife. 2024. PMID: 39133541 Free PMC article. - Wiring the senses: Factors that regulate peripheral axon pathfinding in sensory systems.
Nomdedeu-Sancho G, Alsina B. Nomdedeu-Sancho G, et al. Dev Dyn. 2023 Jan;252(1):81-103. doi: 10.1002/dvdy.523. Epub 2022 Aug 30. Dev Dyn. 2023. PMID: 35972036 Free PMC article. Review. - Frataxin Deficit Leads to Reduced Dynamics of Growth Cones in Dorsal Root Ganglia Neurons of Friedreich's Ataxia YG8sR Model: A Multilinear Algebra Approach.
Muñoz-Lasso DC, Mollá B, Sáenz-Gamboa JJ, Insuasty E, de la Iglesia-Vaya M, Pook MA, Pallardó FV, Palau F, Gonzalez-Cabo P. Muñoz-Lasso DC, et al. Front Mol Neurosci. 2022 Jun 13;15:912780. doi: 10.3389/fnmol.2022.912780. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35769335 Free PMC article. - Growth Factors as Axon Guidance Molecules: Lessons From in vitro Studies.
Onesto MM, Short CA, Rempel SK, Catlett TS, Gomez TM. Onesto MM, et al. Front Neurosci. 2021 May 21;15:678454. doi: 10.3389/fnins.2021.678454. eCollection 2021. Front Neurosci. 2021. PMID: 34093120 Free PMC article. Review. - Current Advances in Comprehending Dynamics of Regenerating Axons and Axon-Glia Interactions after Peripheral Nerve Injury in Zebrafish.
Gonzalez D, Allende ML. Gonzalez D, et al. Int J Mol Sci. 2021 Mar 2;22(5):2484. doi: 10.3390/ijms22052484. Int J Mol Sci. 2021. PMID: 33801205 Free PMC article. Review.
References
- Harrison RG. Experimental investigation of the development of the sense organs of the lateral line in Amphibians (translated from German) Arch Mikrosk Anat. 1904;63:35–149.
- Stone LS. Further experimental studies of the development of the lateral-line sense organs in amphibians observed in living preparations. J Comp Neurol. 1937;68:83–115.
- Ghysen A, Dambly-Chaudière C. The lateral line microcosmos. Genes Dev. 2007;21:2118–2130. - PubMed
- Grant KA, Raible DW, Piotrowski T. Regulation of latent sensory hair cell precursors by glia in the zebrafish lateral line. Neuron. 2005;45:69–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials