Clinical implications of the cancer genome - PubMed (original) (raw)
Review
Clinical implications of the cancer genome
Laura E Macconaill et al. J Clin Oncol. 2010.
Abstract
Cancer is a disease of the genome. Most tumors harbor a constellation of structural genomic alterations that may dictate their clinical behavior and treatment response. Whereas elucidating the nature and importance of these genomic alterations has been the goal of cancer biologists for several decades, ongoing global genome characterization efforts are revolutionizing both tumor biology and the optimal paradigm for cancer treatment at an unprecedented scope. The pace of advance has been empowered, in large part, through disruptive technological innovations that render complete cancer genome characterization feasible on a large scale. This article highlights cardinal biologic and clinical insights gleaned from systematic cancer genome characterization. We also discuss how the convergence of cancer genome biology, technology, and targeted therapeutics articulates a cohesive framework for the advent of personalized cancer medicine.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
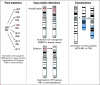
Fig 1.
The major classes of genomic alterations that give rise to cancer. TS, tumor suppressor; CML, chronic myelogenous leukemia.
Fig 2.
Interrogating molecular alterations in cancer. Genomic alterations that give rise to cancers occur at both the RNA and DNA level. Interrogation of the many types of changes and consequent pathway dysregulation has been restricted to date, in part as a result of limiting technologies. Massively parallel sequencing is one emerging technology that enables the myriad cancer-causing alterations to be interrogated on a tumor-by-tumor basis. FISH, fluorescent in situ hybridization; PCR, polymerase chain reaction.
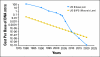
Fig 3.
Advances in massively parallel technologies have dramatically reduced the cost of sequencing. The blue line shows the exponential decrease in cost (US dollars) of sequencing as a function of time. The extrapolated timeline represents human whole-genome sequencing for US$100. The yellow line represents Moore's law—the doubling of computer instructions-per-second (IPS) per dollar every 18 months—indicated as a decrease in cost as a function of time (US$/IPS).
Fig 4.
Schema of personalized medicine. A genome-based vision for personalized cancer medicine will require a paradigm shift in both diagnosis and treatment. Traditionally, tumors are classified by site of origin. In the future, tumor nucleic acid will be profiled for a wide array of genomic alterations with a view to specific, tailored treatment options for each individual patient.
Similar articles
- Existing and emerging technologies for tumor genomic profiling.
MacConaill LE. MacConaill LE. J Clin Oncol. 2013 May 20;31(15):1815-24. doi: 10.1200/JCO.2012.46.5948. Epub 2013 Apr 15. J Clin Oncol. 2013. PMID: 23589546 Free PMC article. Review. - An integrated framework for reporting clinically relevant biomarkers from paired tumor/normal genomic and transcriptomic sequencing data in support of clinical trials in personalized medicine.
Nasser S, Kurdolgu AA, Izatt T, Aldrich J, Russell ML, Christoforides A, Tembe W, Keifer JA, Corneveaux JJ, Byron SA, Forman KM, Zuccaro C, Keats JJ, Lorusso PM, Carpten JD, Trent JM, Craig DW. Nasser S, et al. Pac Symp Biocomput. 2015:56-67. Pac Symp Biocomput. 2015. PMID: 25592568 - Defining precision: The precision medicine initiative trials NCI-MPACT and NCI-MATCH.
Coyne GO, Takebe N, Chen AP. Coyne GO, et al. Curr Probl Cancer. 2017 May-Jun;41(3):182-193. doi: 10.1016/j.currproblcancer.2017.02.001. Epub 2017 Feb 11. Curr Probl Cancer. 2017. PMID: 28372823 - Molecular profiling for personalized cancer care.
Ashfaq R. Ashfaq R. Clin Exp Metastasis. 2012 Oct;29(7):653-5. doi: 10.1007/s10585-012-9483-3. Epub 2012 Jun 8. Clin Exp Metastasis. 2012. PMID: 22678423 Free PMC article. - Genomic Analysis of Childhood Brain Tumors: Methods for Genome-Wide Discovery and Precision Medicine Become Mainstream.
Mack SC, Northcott PA. Mack SC, et al. J Clin Oncol. 2017 Jul 20;35(21):2346-2354. doi: 10.1200/JCO.2017.72.9921. Epub 2017 Jun 22. J Clin Oncol. 2017. PMID: 28640705 Review.
Cited by
- A framework for target discovery in rare cancers.
Li B, Sadagopan A, Li J, Wu Y, Cui Y, Konda P, Weiss CN, Choueiri TK, Doench JG, Viswanathan SR. Li B, et al. bioRxiv [Preprint]. 2024 Oct 25:2024.10.24.620074. doi: 10.1101/2024.10.24.620074. bioRxiv. 2024. PMID: 39484513 Free PMC article. Preprint. - Universal Genetic Testing for Newly Diagnosed Invasive Breast Cancer.
Rezoug Z, Totten SP, Szlachtycz D, Atayan A, Mohler K, Albert S, Feng L, Lemieux Anglin B, Shen Z, Jimenez D, Hamel N, Meti N, Esfahani K, Boileau JF, Prakash I, Basik M, Meterissian S, Tremblay F, Fleiszer D, Anderson D, Chong G, Wong SM, Foulkes WD. Rezoug Z, et al. JAMA Netw Open. 2024 Sep 3;7(9):e2431427. doi: 10.1001/jamanetworkopen.2024.31427. JAMA Netw Open. 2024. PMID: 39226054 Free PMC article. - Drug Repurposing of Selected Antibiotics: An Emerging Approach in Cancer Drug Discovery.
Bano N, Parveen S, Saeed M, Siddiqui S, Abohassan M, Mir SS. Bano N, et al. ACS Omega. 2024 Jun 13;9(25):26762-26779. doi: 10.1021/acsomega.4c00617. eCollection 2024 Jun 25. ACS Omega. 2024. PMID: 38947816 Free PMC article. Review. - Long-read sequencing for brain tumors.
Shelton WJ, Zandpazandi S, Nix JS, Gokden M, Bauer M, Ryan KR, Wardell CP, Vaske OM, Rodriguez A. Shelton WJ, et al. Front Oncol. 2024 Jun 10;14:1395985. doi: 10.3389/fonc.2024.1395985. eCollection 2024. Front Oncol. 2024. PMID: 38915364 Free PMC article. Review. - Novel metabolic biomarker for early detection and diagnosis to the patients with gastric cardia adenocarcinoma.
Wei MX, Yang Z, Wang PP, Zhao XK, Song X, Xu RH, Hu JF, Zhong K, Lei LL, Han WL, Yang MM, Zhou FY, Han XN, Fan ZM, Li J, Wang R, Li B, Wang LD. Wei MX, et al. Cancer Med. 2024 Mar;13(5):e7015. doi: 10.1002/cam4.7015. Cancer Med. 2024. PMID: 38491808 Free PMC article.
References
- Boveri T. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. J Cell Sci. 2008;121(suppl 1):1–84. - PubMed
- Stehelin D, Varmus HE, Bishop JM, et al. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976;260:170–173. - PubMed
- Parada LF, Tabin CJ, Shih C, et al. Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature. 1982;297:474–478. - PubMed
- Tabin CJ, Bradley SM, Bargmann CI, et al. Mechanism of activation of a human oncogene. Nature. 1982;300:143–149. - PubMed
- Nowell PC, Hungerford DA. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960;25:85–109. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources