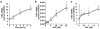Platelet-activating factor induces TLR4 expression in intestinal epithelial cells: implication for the pathogenesis of necrotizing enterocolitis - PubMed (original) (raw)
. 2010 Oct 15;5(10):e15044.
doi: 10.1371/journal.pone.0015044.
Kathrin S Michelsen, Hisae Karahashi, Jing Lu, Fan Jing Meng, Xiaowu Qu, Timothy R Crother, Shervin Rabizadeh, Shuang Chen, Michael S Caplan, Moshe Arditi, Tamas Jilling
Affiliations
- PMID: 20976181
- PMCID: PMC2955554
- DOI: 10.1371/journal.pone.0015044
Platelet-activating factor induces TLR4 expression in intestinal epithelial cells: implication for the pathogenesis of necrotizing enterocolitis
Antoine Soliman et al. PLoS One. 2010.
Abstract
Necrotizing enterocolitis (NEC) is a leading cause of morbidity and mortality in neonatal intensive care units, however its pathogenesis is not completely understood. We have previously shown that platelet activating factor (PAF), bacteria and TLR4 are all important factors in the development of NEC. Given that Toll-like receptors (TLRs) are expressed at low levels in enterocytes of the mature gastrointestinal tract, but were shown to be aberrantly over-expressed in enterocytes in experimental NEC, we examined the regulation of TLR4 expression and signaling by PAF in intestinal epithelial cells using human and mouse in vitro cell lines, and the ex vivo rat intestinal loop model. In intestinal epithelial cell (IEC) lines, PAF stimulation yielded upregulation of both TLR4 mRNA and protein expression and led to increased IL-8 secretion following stimulation with LPS (in an otherwise LPS minimally responsive cell line). PAF stimulation resulted in increased human TLR4 promoter activation in a dose dependent manner. Western blotting and immunohistochemical analysis showed PAF induced STAT3 phosphorylation and nuclear translocation in IEC, and PAF-induced TLR4 expression was inhibited by STAT3 and NFκB Inhibitors. Our findings provide evidence for a mechanism by which PAF augments inflammation in the intestinal epithelium through abnormal TLR4 upregulation, thereby contributing to the intestinal injury of NEC.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
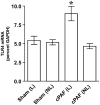
Figure 1. Induction of TLR4 expression by intraluminal PAF.
Adult rat ileal loops were perfused with saline (sham), or with saline +10 µM carbamyl PAF for 4 hrs as indicated. Total RNA was isolated from mucosal scrapings and QRT-PCR was performed to quantify TLR4 and GAPDH mRNA copy numbers. TLR4 mRNA level is shown as % GAPDH. L indicates ileal loop mucosa, NL indicates non loop mucosa. TLR4 mRNA was significantly increased only in the mucosa that was in direct contact with PAF. * p<0.05.
Figure 2. PAF-induced TLR4 expression in intestinal epithelial cells.
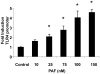
IEC-6 (a and b) and Caco-2 (c) cells were grown in Petri dishes and treated with increasing concentrations of cPAF for for the indicated periods of time (a) or 6 h with 2 µM cPAF (b and c). cDNA copy numbers for TLR4 and GAPDH were quantified using QRT-PCR and TLR4 mRNA levels were expressed as %GAPDH. Treatment with PAF resulted in a dose- and time-dependent increase of TLR4 mRNA. * p<0.01 vs. untreated controls.
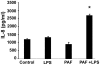
Figure 3. PAF increases IL-8 in intestinal epithelial cells with TLR4 ligand stimulation.
Human IEC (Caco-2) were exposed to PAF 5 µM for 24 h, then stimulated for 18 h with 10 ng/ml LPS. Supernatants were harvested for IL-8 measurement by ELISA. Treatment with PAF caused a priming effect, resulting in LPS-induced IL-8 secretion by Caco-2. * p<0.05 compared to either untreated control, LPS or PAF alone.
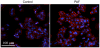
Figure 4. PAF induces TLR4 expression in intestinal epithelial cells.
Human IEC (HT29-Cl19A cells) were grown on glass coverslips and treated without or with cPAF 5 µM for 24 h. Expression of TLR4 (shown in red) was detected using immunofluorescent microscopy (DAPI is blue). Treatment with PAF resulted in an apparently enhanced TLR4 immunoreactivity on the surface of HT29-Cl19A cells. When non-immune serum was used in place of the TLR4 antibody there was no staining detected above background (not shown).
Figure 5. PAF induces TLR4 promoter activation.
HEK293 cells transiently transfected with PAFR, human TLR4 promoter-luciferase and β-galactosidase cDNA were stimulated with cPAF (0–150 nM) for 5 h or empthy vector control. TLR4 promoter luciferase activity was measured with luciferase assay and normalized with β-gal activity. The data are expressed as fold induction of relative light units when compared with transfection of unstimulated controls. This data is representative experiment of three independent experiments performed in triplicate and _y_-error bars indicate SD. *p<0.05 compared to untreated control.
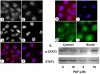
Figure 6. PAF-induced STAT3 translocation to nuclei in IEC-6 cells and in COS-7 cells transfected with the human PAFR.
IEC-6 cells were treated without (a,b,c), or with (d,e,f) 2 µM PAF for 1 h then fixed and processed for STAT3 immunohistochemistry (a,d; c and f red) and nuclei were counterstained with Hoechst dye (b,e; c and f blue). COS-7 cells were first incubated with AD-PAFR for 24 hrs then without (g,h), or with (i,j) 1 µM PAF for 1 h. Triple label immunohistochemistry was performed with anti STAT3 (red), anti PAFR (green) and the DNA binding dye Hoechst (blue). Rat small intestinal cells (IEC-6) were treated as indicated, then Western blots were performed on cytoplasmic and nuclear fractions using antibodies against STAT3 and phorphorylated STAT3 (p-STAT3).
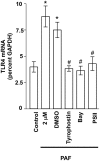
Figure 7. Inhibition of PAF-induced TLR4 expression by STAT3 and NFκB inhibitors.
IEC-6 cells were untreated, treated with PAF (2 µM 4 h), or with PAF (2 µM 4 h) following pretreatment for 1 h with: a) DMSO (vehicle; 1∶1000; all inhibitors were dissolved in DMSO at a 1000× stock), b) Tyrophostin AG490 (25 µM), c) Bay 11–7082 (10 µM), or PSI-II (1 µM). Subsequently, total RNA was isolated and TLR4 mRNA expression was determined using GAPDH reference and quantitative real time PCR. Data shown are mean ±S.E.M. of at least n = 6 in each group. * depicts statistical significance compared to vehicle treated control of p<0.01 and # depicts statistical significance compared to PAF+DMSO of p<0.05 using ANOVA and Tukey's post hoc analysis.
Similar articles
- A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair.
Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. Leaphart CL, et al. J Immunol. 2007 Oct 1;179(7):4808-20. doi: 10.4049/jimmunol.179.7.4808. J Immunol. 2007. PMID: 17878380 - Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis.
Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T Jr, Branca M, Russo A, Gribar SC, Ma C, Hackam DJ. Sodhi CP, et al. Gastroenterology. 2010 Jan;138(1):185-96. doi: 10.1053/j.gastro.2009.09.045. Epub 2009 Sep 26. Gastroenterology. 2010. PMID: 19786028 Free PMC article. - Nucleotide-binding oligomerization domain-2 inhibits toll-like receptor-4 signaling in the intestinal epithelium.
Richardson WM, Sodhi CP, Russo A, Siggers RH, Afrazi A, Gribar SC, Neal MD, Dai S, Prindle T Jr, Branca M, Ma C, Ozolek J, Hackam DJ. Richardson WM, et al. Gastroenterology. 2010 Sep;139(3):904-17, 917.e1-6. doi: 10.1053/j.gastro.2010.05.038. Epub 2010 May 24. Gastroenterology. 2010. PMID: 20580721 Free PMC article. - Toll-Like Receptor-Mediated Intestinal Inflammatory Imbalance in the Pathogenesis of Necrotizing Enterocolitis.
Hackam DJ, Sodhi CP. Hackam DJ, et al. Cell Mol Gastroenterol Hepatol. 2018 Apr 6;6(2):229-238.e1. doi: 10.1016/j.jcmgh.2018.04.001. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 30105286 Free PMC article. Review. - Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch.
Hackam DJ, Good M, Sodhi CP. Hackam DJ, et al. Semin Pediatr Surg. 2013 May;22(2):76-82. doi: 10.1053/j.sempedsurg.2013.01.003. Semin Pediatr Surg. 2013. PMID: 23611610 Free PMC article. Review.
Cited by
- Microbial Metabolites as Ligands to Xenobiotic Receptors: Chemical Mimicry as Potential Drugs of the Future.
Dvořák Z, Li H, Mani S. Dvořák Z, et al. Drug Metab Dispos. 2023 Feb;51(2):219-227. doi: 10.1124/dmd.122.000860. Epub 2022 Oct 2. Drug Metab Dispos. 2023. PMID: 36184080 Free PMC article. Review. - Inflammatory signaling in NEC: Role of NF-κB, cytokines and other inflammatory mediators.
Hunter CJ, De Plaen IG. Hunter CJ, et al. Pathophysiology. 2014 Feb;21(1):55-65. doi: 10.1016/j.pathophys.2013.11.010. Epub 2013 Dec 31. Pathophysiology. 2014. PMID: 24388163 Free PMC article. No abstract available. - Pathogenesis of NEC: Role of the innate and adaptive immune response.
Denning TL, Bhatia AM, Kane AF, Patel RM, Denning PW. Denning TL, et al. Semin Perinatol. 2017 Feb;41(1):15-28. doi: 10.1053/j.semperi.2016.09.014. Epub 2016 Dec 9. Semin Perinatol. 2017. PMID: 27940091 Free PMC article. Review. - Antibiotics increase gut metabolism and antioxidant proteins and decrease acute phase response and necrotizing enterocolitis in preterm neonates.
Jiang P, Jensen ML, Cilieborg MS, Thymann T, Wan JM, Sit WH, Tipoe GL, Sangild PT. Jiang P, et al. PLoS One. 2012;7(9):e44929. doi: 10.1371/journal.pone.0044929. Epub 2012 Sep 13. PLoS One. 2012. PMID: 23028687 Free PMC article. - Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis.
Lu P, Sodhi CP, Hackam DJ. Lu P, et al. Pathophysiology. 2014 Feb;21(1):81-93. doi: 10.1016/j.pathophys.2013.11.007. Epub 2013 Dec 22. Pathophysiology. 2014. PMID: 24365655 Free PMC article.
References
- Caplan MS, MacKendrick W. Inflammatory mediators and intestinal injury. Clin Perinatol. 1994;21:235–246. - PubMed
- Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics. 1999;103:766–771. - PubMed
- Caplan MS, Hedlund E, Adler L, Lickerman M, Hsueh W. The platelet-activating factor receptor antagonist WEB 2170 prevents neonatal necrotizing enterocolitis in rats. J Pediatr Gastroenterol Nutr. 1997;24:296–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI058128/AI/NIAID NIH HHS/United States
- R01 DK062960/DK/NIDDK NIH HHS/United States
- R01DK062960/DK/NIDDK NIH HHS/United States
- R01HD037581/HD/NICHD NIH HHS/United States
- R01AI058128/AI/NIAID NIH HHS/United States
- K08 DK089076/DK/NIDDK NIH HHS/United States
- T32 HD007549/HD/NICHD NIH HHS/United States
- R01 DK062960-03/DK/NIDDK NIH HHS/United States
- R01 HD037581/HD/NICHD NIH HHS/United States
- R01 DK062960-04/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous