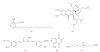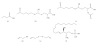Dietary, metabolic, and potentially environmental modulation of the lysine acetylation machinery - PubMed (original) (raw)
Dietary, metabolic, and potentially environmental modulation of the lysine acetylation machinery
Go-Woon Kim et al. Int J Cell Biol. 2010.
Abstract
Healthy lifestyles and environment produce a good state of health. A number of scientific studies support the notion that external stimuli regulate an individual's epigenomic profile. Epigenetic changes play a key role in defining gene expression patterns under both normal and pathological conditions. As a major posttranslational modification, lysine (K) acetylation has received much attention, owing largely to its significant effects on chromatin dynamics and other cellular processes across species. Lysine acetyltransferases and deacetylases, two opposing families of enzymes governing K-acetylation, have been intimately linked to cancer and other diseases. These enzymes have been pursued by vigorous efforts for therapeutic development in the past 15 years or so. Interestingly, certain dietary components have been found to modulate acetylation levels in vivo. Here we review dietary, metabolic, and environmental modulators of the K-acetylation machinery and discuss how they may be of potential value in the context of disease prevention.
Figures
Figure 1
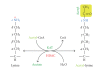
Cartoon illustrating acetylation and deacetylation at the _ε_-amino group of a lysine residue. A KAT is responsible for transfer of an acetyl moiety (in yellow) from acetyl-CoA to the _ε_-group of a lysine residue, whereas an HDAC removes the acetyl group from acetyl lysine, releasing acetate. Note that sirtuins use a catalytic mechanism that is completely different from what is illustrated here for the Rpd3/Hda1 family of deacetylases.
Figure 2
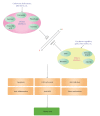
Diagram showing effects of dietary KAT and HDAC inhibition. The capital letter K (in red) refers to a lysine residue and Ac in green refers to acetylation. Different dietary components act on distinct KATs and HDACs to produce differential cellular effects depending on cell types and conditions. On the KAT and HDAC modulators illustrated here, all are small molecules except lunasin and MCP30, which are a 43-residue polypeptide and a 30 kDa protein, respectively. In addition to the inhibitors illustrated here, KAT and HDAC activators, as well as dietary patterns (such as high fat diet, high salt ingestion, and calorie restriction) and environmental factors, may target the acetylation machinery.
Figure 3
Chemical structure of naturally occurring KAT inhibitors. (a) Anacardic acid from cashew nut shell extracts; (b) garcinol from Garcinia indica fruit rind; (c) curcumin from Curcuma longa rhizome; (d) plumbagin from Plumbago rosea root extracts; and (e) spermidine as an endogenous molecule [28, 32, 33]. Some of these compounds have been modified to synthesize more enzyme-specific inhibitors, which are not depicted here [34, 35].
Figure 4
Chemical structure of dietary and endogenous inhibitors of HDACs. (a) butyric acid, acid form of butyrate, a product from dietary fiber fermentation by gut bacteria; (b, c) sulforaphane cysteine and sulforaphane _N_-acetyl-cysteine from cruciferous vegetables; (d, e) allyl mercaptan and diallyl disulfide from garlic; and (f) sphingosine-1-phosphate [–64]. These compounds are either naturally or endogenously occurring, and they act on different members of the Rpd3/Hda1 family. The structures may be used as templates to generate more specific compounds with higher specificity and efficacy.
Similar articles
- Lysine acetylation in obesity, diabetes and metabolic disease.
Iyer A, Fairlie DP, Brown L. Iyer A, et al. Immunol Cell Biol. 2012 Jan;90(1):39-46. doi: 10.1038/icb.2011.99. Epub 2011 Nov 15. Immunol Cell Biol. 2012. PMID: 22083525 Review. - Protein Acetylation in Bacteria.
VanDrisse CM, Escalante-Semerena JC. VanDrisse CM, et al. Annu Rev Microbiol. 2019 Sep 8;73:111-132. doi: 10.1146/annurev-micro-020518-115526. Epub 2019 May 15. Annu Rev Microbiol. 2019. PMID: 31091420 Free PMC article. Review. - A Molecular Perspective on Sirtuin Activity.
Teixeira CSS, Cerqueira NMFSA, Gomes P, Sousa SF. Teixeira CSS, et al. Int J Mol Sci. 2020 Nov 15;21(22):8609. doi: 10.3390/ijms21228609. Int J Mol Sci. 2020. PMID: 33203121 Free PMC article. Review. - K-acetylation and its enzymes: overview and new developments.
Aka JA, Kim GW, Yang XJ. Aka JA, et al. Handb Exp Pharmacol. 2011;206:1-12. doi: 10.1007/978-3-642-21631-2_1. Handb Exp Pharmacol. 2011. PMID: 21879443 Review. - HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention.
Yang XJ, Seto E. Yang XJ, et al. Oncogene. 2007 Aug 13;26(37):5310-8. doi: 10.1038/sj.onc.1210599. Oncogene. 2007. PMID: 17694074 Review.
Cited by
- The remodel of the "central dogma": a metabolomics interaction perspective.
Costa Dos Santos G Jr, Renovato-Martins M, de Brito NM. Costa Dos Santos G Jr, et al. Metabolomics. 2021 May 9;17(5):48. doi: 10.1007/s11306-021-01800-8. Metabolomics. 2021. PMID: 33969452 Free PMC article. Review. - Epigenomics and the microbiota.
Alenghat T. Alenghat T. Toxicol Pathol. 2015 Jan;43(1):101-6. doi: 10.1177/0192623314553805. Epub 2014 Oct 20. Toxicol Pathol. 2015. PMID: 25330924 Free PMC article. - Histone deacetylase 3 coordinates commensal-bacteria-dependent intestinal homeostasis.
Alenghat T, Osborne LC, Saenz SA, Kobuley D, Ziegler CG, Mullican SE, Choi I, Grunberg S, Sinha R, Wynosky-Dolfi M, Snyder A, Giacomin PR, Joyce KL, Hoang TB, Bewtra M, Brodsky IE, Sonnenberg GF, Bushman FD, Won KJ, Lazar MA, Artis D. Alenghat T, et al. Nature. 2013 Dec 5;504(7478):153-7. doi: 10.1038/nature12687. Epub 2013 Nov 3. Nature. 2013. PMID: 24185009 Free PMC article. - Impact of Gut Microbiome on Hypertensive Patients With Low-Salt Intake: Shika Study Results.
Nagase S, Karashima S, Tsujiguchi H, Tsuboi H, Miyagi S, Kometani M, Aono D, Higashitani T, Demura M, Sakakibara H, Yoshida A, Hara A, Nakamura H, Takeda Y, Nambo H, Yoneda T, Okamoto S. Nagase S, et al. Front Med (Lausanne). 2020 Sep 2;7:475. doi: 10.3389/fmed.2020.00475. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32984370 Free PMC article. - Gut indigenous microbiota and epigenetics.
Shenderov BA. Shenderov BA. Microb Ecol Health Dis. 2012 Mar 28;23. doi: 10.3402/mehd.v23i0.17195. eCollection 2012. Microb Ecol Health Dis. 2012. PMID: 23990811 Free PMC article.
References
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. - PubMed
- Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nature Reviews Molecular Cell Biology. 2007;8(4):284–295. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous