Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles - PubMed (original) (raw)
Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles
Mostafa Bakhti et al. J Biol Chem. 2011.
Abstract
Myelin formation is a multistep process that is controlled by a number of different extracellular factors. During the development of the central nervous system (CNS), oligodendrocyte progenitor cells differentiate into mature oligodendrocytes that start to enwrap axons with myelin membrane sheaths after receiving the appropriate signal(s) from the axon or its microenvironment. The signals required to initiate this process are unknown. Here, we show that oligodendrocytes secrete small membrane vesicles, exosome-like vesicles, into the extracellular space that inhibit both the morphological differentiation of oligodendrocytes and myelin formation. The inhibitory effects of exosome-like vesicles were prevented by treatment with inhibitors of actomyosin contractility. Importantly, secretion of exosome-like vesicles from oligodendrocytes was dramatically reduced when cells were incubated by conditioned neuronal medium. In conclusion, our results provide new evidence for small and diffusible oligodendroglial-derived vesicular carriers within the extracellular space that have inhibitory properties on cellular growth. We propose that neurons control the secretion of autoinhibitory oligodendroglial-derived exosomes to coordinate myelin membrane biogenesis.
Figures
FIGURE 1.
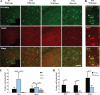
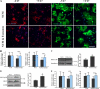
Subcellular localization of PLP in the developing brain of wild-type and shiverer mice. A, immunohistochemistry of brain sections of P7, P14, and P21 wild-type mice for PLP (green) and Lamp-1 (red) as well as of P21 shiverer mice is shown. Colocalization was observed in sections from P7 and P14 but not P21 wild-type mice. Colocalization is found in brain sections from P21 shiverer mice. Bars, 50 μm. B, immunohistochemistry of brain sections of P14 wild-type mice for PLP (green) and Lamp-1 (red) in higher magnification. Bars, 5 μm. C, quantification of PLP and MAG in the cell body of brain sections from P7, P14, and P21 wild-type mice is displayed (n = 30 fields from different animals). D, the comparison of cell body localization of PLP, MOG, and MAG in P21 brain sections of wild-type and shiverer mice is shown (n = 60 fields from different animals). ***, p < 0.001; t test. n.s., not significant.
FIGURE 2.
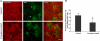
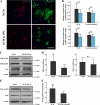
Identification and characterization of exosome-like vesicles secreted from primary cultures of oligodendrocytes. A, the medium of primary oliogodendrocytes cultured for 6–8 days was collected and submitted to sequential centrifugation steps (3000 × g pellet (P3); 4000 × g pellet (P4); 10,000 × g (P10), and 100,000 × g (P100)) as indicated. The resulting pellets of each centrifugation step were analyzed by Western blotting for PLP. B, cell lysates (CL) and 100,000 × g pellets (P100) were analyzed by Western blotting for the indicated proteins. C, the 100,000 × g pellet was prepared and negatively stained with 1% uranyl acetate. Scale bar, 50 nm.
FIGURE 3.
Exosome-like vesicles inhibit myelination. A, oligodendrocytes and neurons were treated 1 day after coculture with exosomes for 4 days. The cells were immunolabeled for β-III-tubulin (red) and MBP (green). Scale bar, 100 μm. B, myelinating oligodendrocytes were quantified by determining the number of MBP-positive cells with multiple parallel processes (arrows) and expressed as the ratio of total MBP-positive cells (n ∼ 500 cells from three independent experiments. *, p < 0.05; t test.
FIGURE 4.
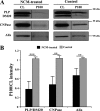
Neuronal conditioned medium reduces PLP, CNPase, and Alix release from oligodendrocytes. A, primary oligodendrocytes were cultured for 6 days, switched to exosome-depleted NCM and exosome-like vesicles prepared 24 h later from the medium by sequential centrifugation steps. B, the amount of PLP, CNPase, and Alix was determined in the cell lysate (CL) and in the exosome fraction (P100). **, p < 0.01; ***, p < 0.001; t test.
FIGURE 5.
Exosome-like vesicles inhibits cell surface expansion of oligodendrocytes. A, oligodendrocytes were treated with 10 μ
m
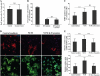
BrdU after 1 day in culture, and the proliferation assay was performed after 24 h (n = 3). B, primary cultures of oligodendrocytes were grown in presence (control) or absence of B27 supplement. Treatment of oligodendrocytes with exosome-like vesicles did not prevent oligodendrocyte cell death (n = 50 confocal images from two independent experiments). C, treatment of oligodendrocytes with NCM for 3 days increased the number of MBP-positive cells. Cotreatment of NCM with exosome-like vesicles did not change the number of MBP-positive cells (n = 50 confocal images from three independent experiments). D and E, primary oligodendrocytes were treated for 3 days with NCM and incubated with exosome-like vesicles during the last 2 days as indicated. Cell surface area of A2B5- and MBP-positive cells was determined as described under “Experimental Procedures.” Treatment with NCM resulted in an increase in cell surface area. Addition of exosome-like vesicles completely prevented the increase in cell surface area (n = 50 confocal images from three independent experiments). ***, p < 0.001; analysis of variance. Scale bar, 100 μm. n.s., not significant.
FIGURE 6.
Inhibition of actomyosin contractility prevents the effects of exosome-like vesicles on cell surface area. A, primary oligodendrocytes were treated for 3 days with NCM and incubated with exosome-like vesicles during the last 2 days in the absence or presence of 10 μ
m
Y27632 (Y27). The cells were immunolabled for A2B5 (red) and MBP (green). Scale bar, 100 μm. B, Y27632 did not change cell surface area of A2B5- and MBP-positive cells treated with only NCM, but the inhibitory effect of exosomes on cell surface expansion was reduced by Y27632. Black column, without Y27632; blue column, with Y27632 (n = 42 confocal images from three independent experiments). C, oligodendrocytes were cultured in NCM for 4 days with or without exosome-like vesicles. Using a pulldown assay, the amount of active RhoA (RhoA-GTP) compared with total RhoA was measured. Treatment with exosome like-vesicles leads to an increase in RhoA activity as compared with the control (n = 3). D, the phosphorylation of MLC2 (Ser-19) was evaluated after treatment of oligodendrocytes with exosome-like vesicles (n = 3). E, blebbistatin (50 μ
m
) did not change cell surface area of A2B5- and MBP-positive cells treated with only NCM, but the inhibitory effect of exosomes on cell surface expansion was reduced by blebbistatin (n = 30 confocal images from two independent experiments). Black column, without blebbistatin; blue column, with blebbistatin (*, p < 0.05; ***, p < 0.001; t test). exo, exosomes.
FIGURE 7.
Exosome-like vesicles affect SFK phosphorylation. A, primary oligodendrocytes were cultured for 3 days in NCM in the absence or presence of 1 μ
m
SFK inhibitor, PP2, for the last 2 days. The cells were immunolabeled for A2B5 (red) and MBP (green). Scale bar, 100 μm. B. Treatment of cells with PP2 leads to a significant reduction in cell surface area of A2B5- and MBP-positive cells. PP2 did not affect cell surface area of oligodendrocytes cultured in the absence of NCM (n = 40 confocal images from three different experiments); black column, without PP2; blue column, with PP2. C, primary oligodendrocytes were treated for 3 days with NCM and either incubated with exosome-like vesicles (Exo) during the last 2 days or not. Cell lysates were analyzed by Western blotting using antibody specific for phosphorylated SFK (Tyr-418, Tyr-529), for Fyn (the major SFK in oligodendrocytes) and for actin as a loading control. D, the quantification of four independent experiments is shown. E, the autophosphorylation of FAK was evaluated using antibody specific for phosphorylated FAK (Tyr-397). F, phosphorylation of FAK is shown as the mean from three independent experiments *, p < 0.05; **, p < 0.01; ***, p < 0.001; t test). n.s., not significant.
Similar articles
- Progressive remodeling of the oligodendrocyte process arbor during myelinogenesis.
Hardy RJ, Friedrich VL Jr. Hardy RJ, et al. Dev Neurosci. 1996;18(4):243-54. doi: 10.1159/000111414. Dev Neurosci. 1996. PMID: 8911764 - Oligodendrocytes support axonal transport and maintenance via exosome secretion.
Frühbeis C, Kuo-Elsner WP, Müller C, Barth K, Peris L, Tenzer S, Möbius W, Werner HB, Nave KA, Fröhlich D, Krämer-Albers EM. Frühbeis C, et al. PLoS Biol. 2020 Dec 22;18(12):e3000621. doi: 10.1371/journal.pbio.3000621. eCollection 2020 Dec. PLoS Biol. 2020. PMID: 33351792 Free PMC article. - CNS myelinogenesis in vitro: time course and pattern of rat oligodendrocyte development.
Asou H, Hamada K, Miyazaki T, Sakota T, Hayashi K, Takeda Y, Marret S, Delpech B, Itoh K, Uyemura K. Asou H, et al. J Neurosci Res. 1995 Mar 1;40(4):519-34. doi: 10.1002/jnr.490400411. J Neurosci Res. 1995. PMID: 7616612 - Oligodendrocytes and the control of myelination in vivo: new insights from the rat anterior medullary velum.
Butt AM, Berry M. Butt AM, et al. J Neurosci Res. 2000 Feb 15;59(4):477-88. doi: 10.1002/(SICI)1097-4547(20000215)59:4<477::AID-JNR2>3.0.CO;2-J. J Neurosci Res. 2000. PMID: 10679786 Review. - Polarity development in oligodendrocytes: sorting and trafficking of myelin components.
Maier O, Hoekstra D, Baron W. Maier O, et al. J Mol Neurosci. 2008 May;35(1):35-53. doi: 10.1007/s12031-007-9024-8. Epub 2008 Jan 3. J Mol Neurosci. 2008. PMID: 18172773 Review.
Cited by
- Oligodendrocyte-lineage cell exocytosis and L-type prostaglandin D synthase promote oligodendrocyte development and myelination.
Pan L, Trimarco A, Zhang AJ, Fujimori K, Urade Y, Sun LO, Taveggia C, Zhang Y. Pan L, et al. Elife. 2023 Feb 13;12:e77441. doi: 10.7554/eLife.77441. Elife. 2023. PMID: 36779701 Free PMC article. - Exosomes-based therapy of stroke, an emerging approach toward recovery.
Seyedaghamiri F, Salimi L, Ghaznavi D, Sokullu E, Rahbarghazi R. Seyedaghamiri F, et al. Cell Commun Signal. 2022 Jul 22;20(1):110. doi: 10.1186/s12964-022-00919-y. Cell Commun Signal. 2022. PMID: 35869548 Free PMC article. Review. - The Role of Extracellular Vesicles in the Developing Brain: Current Perspective and Promising Source of Biomarkers and Therapy for Perinatal Brain Injury.
Gamage TKJB, Fraser M. Gamage TKJB, et al. Front Neurosci. 2021 Sep 24;15:744840. doi: 10.3389/fnins.2021.744840. eCollection 2021. Front Neurosci. 2021. PMID: 34630028 Free PMC article. Review. - A systematic review on the modifications of extracellular vesicles: a revolutionized tool of nano-biotechnology.
Raghav A, Jeong GB. Raghav A, et al. J Nanobiotechnology. 2021 Dec 30;19(1):459. doi: 10.1186/s12951-021-01219-2. J Nanobiotechnology. 2021. PMID: 34965878 Free PMC article.
References
- Baumann N., Pham-Dinh D. (2001) Physiol. Rev. 81, 871–927 - PubMed
- Simons M., Trotter J. (2007) Curr. Opin Neurobiol. 17, 533–540 - PubMed
- Sherman D. L., Brophy P. J. (2005) Nat. Rev. Neurosci. 6, 683–690 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources