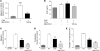Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice - PubMed (original) (raw)
. 2010 Nov;120(11):4129-40.
doi: 10.1172/JCI41732. Epub 2010 Oct 18.
Rory R Koenen, Anna Rueland, Mirko Moreno Zaldivar, Daniel Heinrichs, Hacer Sahin, Petra Schmitz, Konrad L Streetz, Thomas Berg, Nikolaus Gassler, Ralf Weiskirchen, Amanda Proudfoot, Christian Weber, Christian Trautwein, Hermann E Wasmuth
Affiliations
- PMID: 20978355
- PMCID: PMC2964968
- DOI: 10.1172/JCI41732
Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice
Marie-Luise Berres et al. J Clin Invest. 2010 Nov.
Abstract
Activation of hepatic stellate cells in response to chronic inflammation represents a crucial step in the development of liver fibrosis. However, the molecules involved in the interaction between immune cells and stellate cells remain obscure. Herein, we identify the chemokine CCL5 (also known as RANTES), which is induced in murine and human liver after injury, as a central mediator of this interaction. First, we showed in patients with liver fibrosis that CCL5 haplotypes and intrahepatic CCL5 mRNA expression were associated with severe liver fibrosis. Consistent with this, we detected Ccl5 mRNA and CCL5 protein in 2 mouse models of liver fibrosis, induced by either injection of carbon tetrachloride (CCl4) or feeding on a methionine and choline-deficient (MCD) diet. In these models, Ccl5-/- mice exhibited decreased hepatic fibrosis, with reduced stellate cell activation and immune cell infiltration. Transplantation of Ccl5-deficient bone marrow into WT recipients attenuated liver fibrosis, identifying infiltrating hematopoietic cells as the main source of Ccl5. We then showed that treatment with the CCL5 receptor antagonist Met-CCL5 inhibited cultured stellate cell migration, proliferation, and chemokine and collagen secretion. Importantly, in vivo administration of Met-CCL5 greatly ameliorated liver fibrosis in mice and was able to accelerate fibrosis regression. Our results define a successful therapeutic approach to reduce experimental liver fibrosis by antagonizing Ccl5 receptors.
Figures
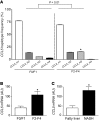
Figure 1. Association of CCL5 with liver fibrosis in humans.
(A) Haplotype analysis of the CCL5 gene in patients with mild (stage F0/F1, 2_n_ = 200) or advanced (stage F2–F4, 2_n_ = 222) liver fibrosis. The overall haplotype distribution is significantly different between patients with mild fibrosis versus subjects with severe fibrosis (P = 0.01). Specifically, the third most common haplotype, CCL5_H3, is significantly more prevalent in individuals with advanced fibrosis (15.4%) compared with patients with mild fibrosis (5.9%, OR 2.83; *P < 0.05). (B) CCL5 mRNA expression is significantly elevated in subjects with advanced HCV-induced fibrosis compared with that of patients with only mild fibrosis (*P < 0.05). (C) The association of CCL5 mRNA expression with liver fibrosis is confirmed in patients with fibrosis due to NASH (*P < 0.05).
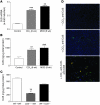
Figure 2. Association of Ccl5 with experimental liver fibrosis in mice.
(A) Ccl5 mRNA expression is significantly increased in total livers of mice treated with either CCl4 for 6 weeks or the MCD diet for 8 weeks (**P < 0.01, ***P < 0.001). (B) The increased expression of Ccl5 is also evident in the protein levels in both models of liver injury (**P < 0.01, ***P < 0.001). (C) Ccl5 protein content is significantly reduced in WT mice that received bone marrow from Ccl5–/– mice (Ccl5–/– → WT) after CCl4 challenge compared with that of WT mice that received WT bone marrow (WT → WT) (**P < 0.01) or Ccl5–/– mice transplanted with WT bone marrow (WT → Ccl5–/–) (#P < 0.05), suggesting that hematopoietic cells are the main source of Ccl5 during CCl4-induced liver fibrosis. (D) Immunohistochemical detection of Ccl5 in the murine liver shows only faint staining in normal liver (original magnification, ×100 [top panel]). The expression of Ccl5 is predominantly increased around blood vessels after treatment with CCl4 (original magnification, ×100 [middle panel]). Costaining with anti-CD3 (T cells) reveals that a significant number of Ccl5-positive cells are also positive for CD3 (original magnification, ×200 [bottom panel]).
Figure 3. Experimental liver fibrosis in _Ccl5_-knockout mice.
(A) Representative Sirius red stainings of WT and Ccl5–/– mice after challenge with CCl4 (original magnification, ×40). Reduced fibrosis in Ccl5–/– mice (n = 12/group) is validated by the significantly lower Sirius red–positive area (***P < 0.001) and decreased hydroxyproline concentrations (*P < 0.05). (B) Ccl5–/– mice also have lower ALT values compared with WT littermates (**P < 0.01). (C) Treatment of Ccl5–/– mice with CCl4 leads to significantly reduced mRNA levels of Col1a1, Tgfb1, Timp1, and Il6 (all with P values of at least < 0.05; *P < 0.05, **P < 0.01, ***P < 0.001), compared with WT mice. (D) Immunohistochemistry demonstrates reduced α-SMA–positive cells within the livers of Ccl5–/– mice compared with WT mice after induction of liver fibrosis (original magnification, ×100). Decreased α-SMA protein expression is also evident in liver lysates of Ccl5–/– mice (representative samples). (E) Ameliorated fibrogenesis in Ccl5–/– mice (n = 12/group) is confirmed in the MCD diet model of liver fibrosis, as shown by representative Sirius red stainings (original magnification, ×40). Decreased deposition of collagen in Ccl5–/– animals is validated by the reduced Sirius red–positive area (***P < 0.001) and lower hydroxyproline concentrations (*P < 0.05). (F) As in the CCl4 model, ALT values are also reduced in Ccl5–/– mice after feeding with the MCD diet (**P < 0.01). (G) Likewise, Col1a1 and Timp1 mRNA is reduced in Ccl5–/– mice compared with their littermates after feedings with MCD diet for 8 weeks (*P < 0.05). (H) Furthermore, Ccl5–/– mice show a trend toward lower hepatic triglyceride levels (P = 0.1).
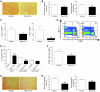
Figure 4. Bone marrow chimeric mice.
(A) WT mice that receive bone marrow from Ccl5–/– mice display significantly reduced liver fibrosis compared with Ccl5–/– or WT animals transplanted with WT bone marrow (n = 7–8/group; original magnification, ×40) (B) The reduced propensity to liver damage of Ccl5–/– → WT mice is also shown by a lower Sirius red–positive area on histology (***P < 0.001), (C) reduced hydroxyproline liver contents (***P < 0.001), and (D) lower ALT values (*P < 0.05). (E) The mRNA expression of fibrosis-related genes, Col1a1, Timp1, and Tgfb1, is also strongly reduced in Ccl5–/– → WT mice compared with that of the other groups (***P < 0.001, **P < 0.01, *P < 0.05).
Figure 5. In vitro evidence for a role of Ccl5 in liver fibrosis.
(A) Migration of stellate cells toward Ccl5 was assessed in Boyden chamber experiments. Stellate cells actively migrate toward Ccl5 (***P < 0.001, compared with medium), which is strongly inhibited by the pretreatment of the cells with Met-CCL5. (B) Ccl5 and Ccl5/Tnf-α stimulation of stellate cells leads to increased secretion of Ccl2 (*P < 0.05, versus unstimulated cells) after 24 hours, which can be significantly inhibited by Met-CCL5 (#P < 0.05, versus Ccl5- and Ccl5/Tnf-α–stimulated cells). Supernatant from activated T cell–enriched splenocyte cultures of WT mice strongly stimulates the migration (C), proliferation (D), and collagen protein secretion (E) of stellate cells. These profibrotic phenotypes of stellate cells are severely blunted by supernatants from either splenocytes of Ccl5–/– mice or pretreatment of stellate cells with Met-CCL5 (**P < 0.01, ***P < 0.001). All in vitro experiments were performed at least twice in quadruplicates.
Figure 6. In vivo inhibition of liver fibrosis by Met-CCL5.
(A) Representative Sirius red stainings of CCl4-treated C57BL/6 WT mice, with a concomitant administration of vehicle or Met-CCL5 (n = 10/group; original magnification, ×40). (B) Reduced fibrosis in Met-CCL5–treated mice is validated by a significantly lower Sirius red–positive area (*P < 0.05) and decreased concentrations of hydroxyproline (**P < 0.01). (C) The reduced fibrotic response in Met-CCL5–treated mice is further evidenced by significantly reduced Col1a1 and Timp1 mRNA levels (*P < 0.05, **P < 0.01). (D) Representative FACS blot of NK1.1 and CD3-positive cell infiltration in vehicle- and Met-CCL5–treated animals, demonstrating a reduced T cell infiltration into the livers of Met-CCL5–treated mice, while numbers of NK and NKT cells are not significantly altered. (E) Statistical analysis of T cell infiltration (FACS) reveals a significantly reduced number of CD3- and, specifically, CD8-positive cells after treatment with Met-CCL5 (***P < 0.001). (F) In addition, numbers of CD68+-positive macrophages are also reduced in Met-CCL5–treated animals compared with those of vehicle-treated mice (*P < 0.05, n = 10/group). (G) The antifibrotic potential of Met-CCL5 is validated in the MCD fibrosis model by histology (original magnification, ×40). (H) Reduced liver damage in Met-CCL5–treated mice is evidenced by a lower Sirius red–positive area in histology (**P < 0.01) and significantly reduced hydroxyproline levels (**P < 0.01) compared with those of vehicle-treated animals.
Figure 7. Met-CCL5 accelerates the regression of liver fibrosis in vivo.
(A) C57BL/6 mice were challenged with CCl4 for 8 weeks to establish advanced liver scarring. Three days after the last the last CCl4 injection (at the peak of fibrosis), mice received either Met-CCL5 or vehicle (n = 8/group) and were assessed for fibrosis regression by histology for an overall duration of 7 days. At day 7, the mice that received Met-CCL5 displayed a significantly reduced residual fibrosis compared with the vehicle-treated group (original magnifications, ×40). (B) The difference between the groups is evidenced by a reduced Sirius red–positive area in the Met-CCL5–treated mice (*P < 0.05) and (C) by significantly lower hydroxyproline contents at the same time point during fibrosis regression (*P < 0.05). (D) Functionally, mRNA expression of Col1a1 and Timp1 is already significantly reduced at day 3, after start of Met-CCL5 or vehicle treatment (*P < 0.05).
Comment in
- RANTES antagonism: a promising approach to treat chronic liver diseases.
Affò S, Bataller R. Affò S, et al. J Hepatol. 2011 Oct;55(4):936-8. doi: 10.1016/j.jhep.2011.04.023. Epub 2011 Jun 25. J Hepatol. 2011. PMID: 21708198 - Anti-chemokine therapy for the treatment of hepatic fibrosis: an attractive approach.
Ramm GA. Ramm GA. Hepatology. 2011 Jul;54(1):354-8. doi: 10.1002/hep.24353. Hepatology. 2011. PMID: 21710472 No abstract available.
Similar articles
- RANTES antagonism: a promising approach to treat chronic liver diseases.
Affò S, Bataller R. Affò S, et al. J Hepatol. 2011 Oct;55(4):936-8. doi: 10.1016/j.jhep.2011.04.023. Epub 2011 Jun 25. J Hepatol. 2011. PMID: 21708198 - Interference with oligomerization and glycosaminoglycan binding of the chemokine CCL5 improves experimental liver injury.
Nellen A, Heinrichs D, Berres ML, Sahin H, Schmitz P, Proudfoot AE, Trautwein C, Wasmuth HE. Nellen A, et al. PLoS One. 2012;7(5):e36614. doi: 10.1371/journal.pone.0036614. Epub 2012 May 4. PLoS One. 2012. PMID: 22574195 Free PMC article. - Steatosis induced CCL5 contributes to early-stage liver fibrosis in nonalcoholic fatty liver disease progress.
Li BH, He FP, Yang X, Chen YW, Fan JG. Li BH, et al. Transl Res. 2017 Feb;180:103-117.e4. doi: 10.1016/j.trsl.2016.08.006. Epub 2016 Aug 31. Transl Res. 2017. PMID: 27639593 - Functional role of CCL5/RANTES for HCC progression during chronic liver disease.
Mohs A, Kuttkat N, Reißing J, Zimmermann HW, Sonntag R, Proudfoot A, Youssef SA, de Bruin A, Cubero FJ, Trautwein C. Mohs A, et al. J Hepatol. 2017 Apr;66(4):743-753. doi: 10.1016/j.jhep.2016.12.011. Epub 2016 Dec 21. J Hepatol. 2017. PMID: 28011329 - Deletion of C-C Motif Chemokine Ligand 5 Worsens Invariant Natural Killer T-Cell-Mediated Hepatitis via Compensatory Up-regulation of CXCR2-Related Chemokine Activity.
Chen L, Gu J, Qian Y, Li M, Qian Y, Xu M, Li J, Wen Y, Xia L, Li J, Xia Q, Kong X, Wu H. Chen L, et al. Cell Mol Gastroenterol Hepatol. 2019;7(3):623-639. doi: 10.1016/j.jcmgh.2018.12.009. Epub 2019 Jan 7. Cell Mol Gastroenterol Hepatol. 2019. PMID: 30630119 Free PMC article.
Cited by
- Unveiling the nexus: pyroptosis and its crucial implications in liver diseases.
Miao Z, Zhang X, Xu Y, Liu Y, Yang Q. Miao Z, et al. Mol Cell Biochem. 2024 Oct 31. doi: 10.1007/s11010-024-05147-1. Online ahead of print. Mol Cell Biochem. 2024. PMID: 39477911 Review. - ATF3-mediated transactivation of CXCL14 in HSCs during liver fibrosis.
Li X, Lin L, Li Y, Zhang W, Lang Z, Zheng J. Li X, et al. Clin Transl Med. 2024 Oct;14(10):e70040. doi: 10.1002/ctm2.70040. Clin Transl Med. 2024. PMID: 39358917 Free PMC article. - Oligonucleotide therapies for nonalcoholic steatohepatitis.
Li S, Xiong F, Zhang S, Liu J, Gao G, Xie J, Wang Y. Li S, et al. Mol Ther Nucleic Acids. 2024 Mar 30;35(2):102184. doi: 10.1016/j.omtn.2024.102184. eCollection 2024 Jun 11. Mol Ther Nucleic Acids. 2024. PMID: 38665220 Free PMC article. Review. - Emerging therapies for MASLD and their impact on plasma lipids.
Nguyen M, Asgharpour A, Dixon DL, Sanyal AJ, Mehta A. Nguyen M, et al. Am J Prev Cardiol. 2024 Feb 5;17:100638. doi: 10.1016/j.ajpc.2024.100638. eCollection 2024 Mar. Am J Prev Cardiol. 2024. PMID: 38375066 Free PMC article. - Time-Restricted Feeding Ameliorates Methionine-Choline Deficient Diet-Induced Steatohepatitis in Mice.
Jung IR, Ahima RS, Kim SF. Jung IR, et al. Int J Mol Sci. 2024 Jan 23;25(3):1390. doi: 10.3390/ijms25031390. Int J Mol Sci. 2024. PMID: 38338668 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous