Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor - PubMed (original) (raw)
Clinical Trial
. 2010 Oct 28;363(18):1727-33.
doi: 10.1056/NEJMoa1007056.
David R D'Adamo, Jason L Hornick, Paola Dal Cin, Cristina R Antonescu, Suresh C Jhanwar, Marc Ladanyi, Marzia Capelletti, Scott J Rodig, Nikhil Ramaiya, Eunice L Kwak, Jeffrey W Clark, Keith D Wilner, James G Christensen, Pasi A Jänne, Robert G Maki, George D Demetri, Geoffrey I Shapiro
Affiliations
- PMID: 20979472
- PMCID: PMC3014292
- DOI: 10.1056/NEJMoa1007056
Clinical Trial
Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor
James E Butrynski et al. N Engl J Med. 2010.
Abstract
Inflammatory myofibroblastic tumor (IMT) is a distinctive mesenchymal neoplasm characterized by a spindle-cell proliferation with an inflammatory infiltrate. Approximately half of IMTs carry rearrangements of the anaplastic lymphoma kinase (ALK) locus on chromosome 2p23, causing aberrant ALK expression. We report a sustained partial response to the ALK inhibitor crizotinib (PF-02341066, Pfizer) in a patient with ALK-translocated IMT, as compared with no observed activity in another patient without the ALK translocation. These results support the dependence of ALK-rearranged tumors on ALK-mediated signaling and suggest a therapeutic strategy for genomically identified patients with the aggressive form of this soft-tissue tumor. (Funded by Pfizer and others; ClinicalTrials.gov number, NCT00585195.).
Figures
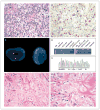
Figure 1. Histologic, Immunohistochemical, and Molecular Analyses of IMT Samples from Patient 1
A sample of the inflammatory myofibroblastic tumor (IMT) obtained on biopsy shows epithelioid cells containing vesicular nuclei, prominent nucleoli, and amphophilic cytoplasm embedded in a myxoid stroma containing prominent neutrophils (Panel A, hematoxylin and eosin). Immunohistochemical analysis for ALK shows positive staining in tumor cells, with a nuclear membrane pattern (Panel B). Dual-color fluorescence in situ hybridization (FISH) shows rearrangement of centromeric (green) and telomeric (orange) probes flanking the ALK locus at 2p23 (Panel C). Gel electrophoresis of polymerase-chain-reaction (PCR) products after reverse-transcriptase PCR (RT-PCR) is shown for primers directed at known ALK translocation partners in IMT, including CARS, CLTC, RANBP2, ATIC, SEC31L1 (which generates both long-form [L] and short-form [S] fusion transcripts), TPM3, and TPM4, (Panel D). Only RANBP2-ALK primers produce an amplification product in the presence of (but not in the absence of) reverse transcriptase. Sequencing of the PCR product confirmed that RANBP2 exon 18 is fused in frame with ALK exon 20 (Panel E). Sections from progressing tumor masses in the liver (Panel F) and perirectal region (Panel G), which were resected after approximately 8 months of crizotinib administration, show histologic heterogeneity, with cellular areas similar in appearance to the initial biopsy sample but also revealing extensive areas suggestive of a treatment effect, with foci of tumor-cell necrosis in the liver sample and marked stromal hyalinization in the perirectal sample (hematoxylin and eosin in Panels F and G). Methods for immunostaining, FISH, and RT-PCR are described in the Supplementary Appendix, available with the full text of this article at NEJM.org.
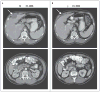
Figure 2. CT Scans Showing the Response to Crizotinib in Patient 1
The baseline abdominal CT scan shows a hepatic mass measuring 4.8 by 3.3 cm (top) and one of several mesenteric masses measuring 3.8 by 3.3 cm (bottom) (Panel A, arrows). After 13 weeks of treatment with crizotinib, the hepatic and mesenteric masses measured 2.3 by 0.8 cm and 1.3 by 1.2 cm, respectively (Panel B, arrows). In October 2008, these masses measured 3.6 by 2.2 cm and 0.5 by 0.5 cm, respectively (not shown), indicating that the hepatic mass had regrown, despite a continued response, according to the Response Evaluation Criteria in Solid Tumors.
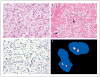
Figure 3. Histologic and Immunohistochemical Analyses of IMT Samples from Patient 2
A sample of the inflammatory myofibroblastic tumor (IMT) obtained on biopsy shows ganglion-like, plump epithelioid cells with abundant eosinophilic cytoplasm and nuclei with open chromatic and prominent nucleoli; scattered small lymphocytes are in the background (Panel A, hematoxylin and eosin). A different area of the tumor shows a prominent inflammatory component that is composed primarily of plasma cells, dense fibrous stroma, and scattered plump spindle and ganglion-like cells (Panel B, arrows; hematoxylin and eosin). Results of immunostaining for ALK are negative (Panel C), and FISH analysis shows no ALK rearrangement (Panel D).
Comment in
- Crizotinib--latest champion in the cancer wars?
Hallberg B, Palmer RH. Hallberg B, et al. N Engl J Med. 2010 Oct 28;363(18):1760-2. doi: 10.1056/NEJMe1010404. N Engl J Med. 2010. PMID: 20979477 No abstract available. - ALK and resistance.
[No authors listed] [No authors listed] Nat Rev Cancer. 2010 Dec;10(12):817. doi: 10.1038/nrc2976. Nat Rev Cancer. 2010. PMID: 21155181 No abstract available. - More on crizotinib.
Orenstein JM. Orenstein JM. N Engl J Med. 2011 Feb 24;364(8):777; author reply 778-9. doi: 10.1056/NEJMc1013325. N Engl J Med. 2011. PMID: 21345112 No abstract available.
Similar articles
- Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer.
Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW, Iafrate AJ. Kwak EL, et al. N Engl J Med. 2010 Oct 28;363(18):1693-703. doi: 10.1056/NEJMoa1006448. N Engl J Med. 2010. PMID: 20979469 Free PMC article. Clinical Trial. - Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study.
Mossé YP, Lim MS, Voss SD, Wilner K, Ruffner K, Laliberte J, Rolland D, Balis FM, Maris JM, Weigel BJ, Ingle AM, Ahern C, Adamson PC, Blaney SM. Mossé YP, et al. Lancet Oncol. 2013 May;14(6):472-80. doi: 10.1016/S1470-2045(13)70095-0. Epub 2013 Apr 16. Lancet Oncol. 2013. PMID: 23598171 Free PMC article. Clinical Trial. - Neoadjuvant crizotinib in advanced inflammatory myofibroblastic tumour with ALK gene rearrangement.
Rafee S, Elamin YY, Joyce E, Toner M, Flavin R, McDermott R, Sheehy N, Hennessy B, O'Byrne K, Gleeson N, Osman N. Rafee S, et al. Tumori. 2015 Apr 28;101(2):e35-9. doi: 10.5301/tj.5000245. Tumori. 2015. PMID: 25744866 - Crizotinib, a small-molecule dual inhibitor of the c-Met and ALK receptor tyrosine kinases.
Rodig SJ, Shapiro GI. Rodig SJ, et al. Curr Opin Investig Drugs. 2010 Dec;11(12):1477-90. Curr Opin Investig Drugs. 2010. PMID: 21154129 Review. - Crizotinib for the treatment of patients with advanced non-small cell lung cancer.
Bowles DW, Weickhardt AJ, Doebele RC, Camidge DR, Jimeno A. Bowles DW, et al. Drugs Today (Barc). 2012 Apr;48(4):271-82. doi: 10.1358/dot.2012.48.4.1769835. Drugs Today (Barc). 2012. PMID: 22536569 Review.
Cited by
- Inflammatory myofibroblastic tumours of the duodenopancreaticobiliary tract.
Shah PA, Babu R, Ramaswamy V, G SK. Shah PA, et al. BMJ Case Rep. 2021 May 6;14(5):e240833. doi: 10.1136/bcr-2020-240833. BMJ Case Rep. 2021. PMID: 33958358 Free PMC article. - Changing paradigm of cancer therapy: precision medicine by next-generation sequencing.
Xue Y, Wilcox WR. Xue Y, et al. Cancer Biol Med. 2016 Mar;13(1):12-8. doi: 10.28092/j.issn.2095-3941.2016.0003. Cancer Biol Med. 2016. PMID: 27144059 Free PMC article. - Role of genetic and molecular profiling in sarcomas.
Norberg SM, Movva S. Norberg SM, et al. Curr Treat Options Oncol. 2015 May;16(5):24. doi: 10.1007/s11864-015-0339-3. Curr Treat Options Oncol. 2015. PMID: 25939540 Review. - FGFR2 is overexpressed in myxoid liposarcoma and inhibition of FGFR signaling impairs tumor growth in vitro.
Künstlinger H, Fassunke J, Schildhaus HU, Brors B, Heydt C, Ihle MA, Mechtersheimer G, Wardelmann E, Büttner R, Merkelbach-Bruse S. Künstlinger H, et al. Oncotarget. 2015 Aug 21;6(24):20215-30. doi: 10.18632/oncotarget.4046. Oncotarget. 2015. PMID: 26036639 Free PMC article. - Recurrent fusion of the genes FN1 and ALK in gastrointestinal leiomyomas.
Panagopoulos I, Gorunova L, Lund-Iversen M, Lobmaier I, Bjerkehagen B, Heim S. Panagopoulos I, et al. Mod Pathol. 2016 Nov;29(11):1415-1423. doi: 10.1038/modpathol.2016.129. Epub 2016 Jul 29. Mod Pathol. 2016. PMID: 27469327
References
- Meis JM, Enzinger FM. Inflammatory fibrosarcoma of the mesentery and retro-peritoneum: a tumor closely simulating inflammatory pseudotumor. Am J Surg Pathol. 1991;15:1146–56. - PubMed
- Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor): a clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–72. - PubMed
- Pettinato G, Manivel JC, De Rosa N, Dehner LP. Inflammatory myofibroblastic tumor (plasma cell granuloma): clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations. Am J Clin Pathol. 1990;94:538–46. - PubMed
- Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61:428–37. - PubMed
- Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14:569–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC2-CA148260/CA/NCI NIH HHS/United States
- R01-CA136851/CA/NCI NIH HHS/United States
- RC2 CA148260/CA/NCI NIH HHS/United States
- P01-CA47179/CA/NCI NIH HHS/United States
- P01 CA047179-19/CA/NCI NIH HHS/United States
- R01 CA136851/CA/NCI NIH HHS/United States
- P01 CA047179/CA/NCI NIH HHS/United States
- R01 CA136851-02/CA/NCI NIH HHS/United States
- RC2 CA148260-02/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous