Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study - PubMed (original) (raw)
Clinical Trial
doi: 10.1093/neuonc/noq142. Epub 2010 Oct 27.
P Hau, G Stockhammer, N K Venkataramana, A K Mahapatra, A Suri, A Balasubramaniam, S Nair, V Oliushine, V Parfenov, I Poverennova, M Zaaroor, P Jachimczak, S Ludwig, S Schmaus, H Heinrichs, K-H Schlingensiepen; Trabedersen Glioma Study Group
Affiliations
- PMID: 20980335
- PMCID: PMC3018908
- DOI: 10.1093/neuonc/noq142
Clinical Trial
Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study
U Bogdahn et al. Neuro Oncol. 2011 Jan.
Abstract
This randomized, open-label, active-controlled, dose-finding phase IIb study evaluated the efficacy and safety of trabedersen (AP 12009) administered intratumorally by convection-enhanced delivery compared with standard chemotherapy in patients with recurrent/refractory high-grade glioma. One hundred and forty-five patients with central reference histopathology of recurrent/refractory glioblastoma multiforme (GBM) or anaplastic astrocytoma (AA) were randomly assigned to receive trabedersen at doses of 10 or 80 µM or standard chemotherapy (temozolomide or procarbazine/lomustine/vincristine). Primary endpoint was 6-month tumor control rate, and secondary endpoints included response at further timepoints, survival, and safety. Six-month tumor control rates were not significantly different in the entire study population (AA and GBM). Prespecified AA subgroup analysis showed a significant benefit regarding the 14-month tumor control rate for 10 µM trabedersen vs chemotherapy (p= .0032). The 2-year survival rate had a trend for superiority for 10 µM trabedersen vs chemotherapy (p = .10). Median survival for 10 µM trabedersen was 39.1 months compared with 35.2 months for 80 µM trabedersen and 21.7 months for chemotherapy (not significant). In GBM patients, response and survival results were comparable among the 3 arms. Exploratory analysis on GBM patients aged ≤55 years with Karnofsky performance status >80% at baseline indicated a 3-fold survival at 2 and 3 years for 10 µM trabedersen vs chemotherapy. The frequency of patients with related or possibly drug-related adverse events was higher with standard chemotherapy (64%) than with 80 µM trabedersen (43%) and 10 µM trabedersen (27%). Superior efficacy and safety for 10 µM trabedersen over 80 µM trabedersen and chemotherapy and positive risk-benefit assessment suggest it as the optimal dose for further clinical development in high-grade glioma.
Figures
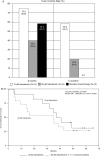
Fig. 1.
Efficacy results in patients with AA (primary efficacy population, _n_= 39). (A) Tumor control rates (complete response + partial response + stable disease) at months 6 and 14. *Comparison between 10 µM trabedersen and standard chemotherapy: _p_= .0032. (B) Kaplan–Meier plot for patients treated with 10 µM trabedersen (_n_= 12) vs 80 µM trabedersen (_n_= 15). (C) Kaplan–Meier plot for patients treated with 10 µM trabedersen (_n_= 12) vs standard chemotherapy (_n_= 12). (D) Overall survival rates.
Fig. 1.
Efficacy results in patients with AA (primary efficacy population, _n_= 39). (A) Tumor control rates (complete response + partial response + stable disease) at months 6 and 14. *Comparison between 10 µM trabedersen and standard chemotherapy: _p_= .0032. (B) Kaplan–Meier plot for patients treated with 10 µM trabedersen (_n_= 12) vs 80 µM trabedersen (_n_= 15). (C) Kaplan–Meier plot for patients treated with 10 µM trabedersen (_n_= 12) vs standard chemotherapy (_n_= 12). (D) Overall survival rates.
Comment in
- Trabedersen to target transforming growth factor-beta: when the journey is not the reward, in reference to Bogdahn et al. (Neuro-Oncology 2011;13:132-142).
Wick W, Weller M. Wick W, et al. Neuro Oncol. 2011 May;13(5):559-60; author reply 561-2. doi: 10.1093/neuonc/nor046. Neuro Oncol. 2011. PMID: 21558079 Free PMC article. No abstract available.
References
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi:10.1056/NEJMoa043330. - DOI - PubMed
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi:10.1016/S1470-2045(09)70025-7. - DOI - PubMed
- Chang SM, Theodosopoulos P, Lamborn K, et al. Temozolomide in the treatment of recurrent malignant glioma. Cancer. 2004;100:605–611. doi:10.1002/cncr.11949. - DOI - PubMed
- Fontana A, Bodmer S, Frei K, Malipiero U, Siepl C. Expression of TGF-beta2 in human glioblastoma: a role in resistance to immune rejection? CIBA Found Symp. 1991;157:232–241. - PubMed
- Maxwell M, Galanopoulos T, Neville-Golden J, Antoniades HN. Effect of the expression of transforming growth factor-beta 2 in primary human glioblastomas on immunosuppression and loss of immune surveillance. J Neurosurg. 1992;76:799–804. doi:10.3171/jns.1992.76.5.0799. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical