Regulation of benzo[a]pyrene-mediated DNA- and glutathione-adduct formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in human lung cells - PubMed (original) (raw)
. 2011 Jan 14;24(1):89-98.
doi: 10.1021/tx100297z. Epub 2010 Oct 28.
Affiliations
- PMID: 21028851
- PMCID: PMC3021323
- DOI: 10.1021/tx100297z
Free PMC article
Regulation of benzo[a]pyrene-mediated DNA- and glutathione-adduct formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in human lung cells
Stacy L Gelhaus et al. Chem Res Toxicol. 2011.
Free PMC article
Abstract
Environmental carcinogens, such as polycyclic aromatic hydrocarbons (PAHs), require metabolic activation to DNA-reactive metabolites in order to exert their tumorigenic effects. Benzo[a]pyrene (B[a]P), a prototypic PAH, is metabolized by cytochrome P450 (P450) 1A1/1B1 and epoxide hydrolase to (-)-B[a]P-7,8-dihydro-7,8-diol (B[a]P-7,8-dihydrodiol). B[a]P-7,8-dihydrodiol then undergoes further P4501A1/1B1-mediated metabolism to the ultimate carcinogen, (+)-anti-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro-B[a]P (B[a]PDE), which forms DNA-adducts primarily with 2'-deoxyguanosine (dGuo) to form (+)-anti-trans-B[a]PDE-N(2)-dGuo (B[a]PDE-dGuo) in DNA. Pretreatment of cells with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is known to induce P4501A1/1B1 mRNA expression through the aryl hydrocarbon receptor (AhR) pathway. This causes increased B[a]PDE-dGuo formation in liver cells. In contrast, TCDD induction of H358 lung cells surprisingly caused a decrease in (-)-B[a]P-7,8-dihydrodiol-mediated (+)-B[a]PDE-dGuo adduct formation when compared with the non-TCDD-induced cells. Furthermore, treatment of the TCDD-induced cells with (±)-B[a]PDE also resulted in decreased (+)-B[a]PDE-dGuo adduct formation when compared with the non-TCDD-induced cells. These data suggested that it was a detoxification pathway that had been up-regulated rather than an activation pathway that had been down-regulated. LC-MS was used to analyze B[a]PDE-dGuo and B[a]PDE-GSH-adducts in H358 lung and HepG2 liver cells. There was a significant increase in the (-)-B[a]PDE-GSH-adduct with high enantiomeric excess after treatment of the TCDD-induced H358 cells with (±)-B[a]PDE when compared with the noninduced cells. This could explain why increased activation of (-)-B[a]P-7,8-dihydrodiol through TCDD up-regulation of P4501A1/1B1 did not lead to increased (+)-B[a]PDE-dGuo adducts in the H358 lung cells. In addition, TCDD did not induce B[a]PDE-GSH-adduct formation in HepG2 liver cells. (±)-B[a]PDE-GSH-adducts were formed at much lower levels in both TCDD-induced and noninduced HepG2 cells when compared with (-)-B[a]PDE-GSH-adducts in the H358 lung cells. Therefore, our study has revealed that there is a subtle balance between activation and detoxification of B[a]P in lung-derived compared with liver-derived cells and that this determines how much DNA damage occurs.
Figures
Scheme 1. Metabolism of B[_a_]P in Human Lung Cells
Figure 1
(−)-B[_a_]P-7,8-dihydrodiol concentration dependence. The levels of (+)-B[_a_]PDE-dGuo DNA-adducts formed in H358 cells with increasing concentrations of (−)-B[a]P-7,8-diol (0.1, 0.5, 1.0, and 2.0 μM) in 24 h with and without 10 nM TCDD pretreatment for 24 h. Experiments were conducted in triplicate, and the values are expressed as the means ± SEM. B[_a_]PDE-dGuo levels were significantly higher (p < 0.05) in H358 cells not treated with TCDD.
Figure 2
LC-MRM/MS analysis of (−)-B[_a_]P-7,8-dihydrodiol in H358 cells. Analyses were conducted after a 6 h incubation of H358 cells treated with 2 μM (−)-B[a]P-7,8-dihydrodiol with and without 10 nM TCDD pretreatment for 24 h. The ratio of (−)-B[_a_]P-7,8-dihydrodiol to the [13C2]-B[_a_]P-7,8-dihydrodiol internal standard was calculated for experiments in triplicate. Experiments were conducted in triplicate, and the values are expressed as the means ± SEM. The ratio for H358 cells pretreated with 10 nM TCDD for 24 h was significantly lower (p < 0.05) than that of H358 cells that were not pretreated with TCDD.
Figure 3
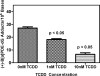
TCDD concentration dependence. H358 cells were pretreated with TCDD (0, 1.0, and 10 nM) for 24 h and treated with 1.0 μM (−)-B[_a_]P-7,8-dihydrodiol for 24 h. Experiments were conducted in triplicate, and the values are expressed as the means ± SEM. As the concentration of TCDD increased, the level of (+)-B[_a_]PDE-dGuo decreased significantly (p < 0.05).
Figure 4
Microarray expression. The fold change in mRNA depending upon the various cell treatments: DMSO, 10 nM TCDD (48 h), 2 μM (−)-B[_a_]P-7,8-dihydrodiol (24 h), and 10 nM TCDD (48 h) with 2 μM (−)-B[_a_]P-7,8-dihydrodiol (24 h). (A) CYP1A1. (B) CYP1B1.
Figure 5
Measurement of (+)-B[_a_]PDE-dGuo H358 cells treated with 2 μM (±)-B[_a_]PDE with and without 10 nM TCDD pretreatment. (+)-B[_a_]PDE-dGuo formation with TCDD-induced cells was decreased compared with that of the noninduced cells.
Figure 6
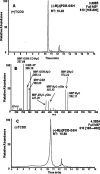
LC-MS/MS analysis of B[_a_]PDE-GSH-adducts in (±)-B[_a_]PDE-treated H358 cells. (A) LC-MS/MS chromatogram of the intracellular (−)-B[_a_]PDE-GSH-adduct in TCDD-induced H358 cells after a 4 h incubation. (B) Product ion spectrum of the (−)-B[_a_]PDE-GSH-adduct (MH+, m/z 610) in TCDD-induced H358 cells. (C) LC-MS/MS chromatogram of the intracellular (−)-B[_a_]PDE-GSH-adduct in noninduced H358 cells after a 4 h incubation. H358 cells pretreated with 10 nM TCDD for 24 h had increased levels of (−)-B[_a_]PDE-GSH-adducts.
Figure 7
LC-MRM/MS analysis of B[_a_]PDE-GSH-adducts in (±)-B[_a_]PDE-treated H358 and HepG2 cells with 10 nM TCDD pretreatment for 24 h. (A) Intracellular (−)-B[_a_]PDE-GSH-adducts in TCDD-induced H358 cells after a 4 h incubation. (B) Product ion spectrum of the (+)-B[_a_]PDE-GSH-adduct in HepG2-induced cells (MH+, m/z 610). (C) Intracellular (±)-B[_a_]PDE-GSH-adducts in TCDD-induced HepG2 cells after a 4 h incubation.
Figure 8
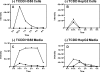
B[_a_]PDE-GSH-adducts in H358 and HepG2 cells and media after TCDD induction. H358 and HepG2 cells were treated with (±)-B[_a_]PDE for 30 min, 1, 2, 4, and 6 h with 10 nM TCDD pretreatment for 24 h and GSH-adducts quantified by LC-MS. (A) Intracellular (±)-B[_a_]PDE-GSH-adducts in H358 cells. (B) Intracellular (±)-B[_a_]PDE-GSH-adducts in HepG2 cells. (C) (±)-B[_a_]PDE-GSH-adducts in H358 cell media. (D) (±)-B[_a_]PDE-GSH-adducts in HepG2 cell media. Analyses were conducted in duplicate, and the mean values are shown. (−)-B[_a_]PDE-GSH-adduct (solid triangle); (+)-B[_a_]PDE-GSH-adduct (solid square).
Figure 9
B[_a_]PDE-GSH-adducts in H358 and HepG2 cells and media with no TCDD induction. H358 and HepG2 cells were treated with (±)-B[_a_]PDE for 30 min, 1, 2, 4, and 6 h without TCDD pretreatment for 24 h and GSH-adduct formation quantified by LC-MS. (A) Intracellular (±)-B[_a_]PDE-GSH-adducts in H358 cells. (B) Intracellular (±)-B[_a_]PDE-GSH-adducts in HepG2 cells. (C) (±)-B[_a_]PDE-GSH-adducts in H358 cell media. (D) (±)-B[_a_]PDE-GSH-adducts HepG2 cell media. Analyses were conducted in duplicate, and the mean values are shown. (−)-B[_a_]PDE-GSH-adduct (solid triangle); (+)-B[_a_]PDE-GSH-adduct (solid square).
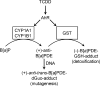
Scheme 2. Activation of B[_a_]P and Detoxification of the Resulting B[_a_]PDE in Human Lung Cells
TCDD induces P450s 1A1 and 1B1, which activate B[_a_]P to the reactive intermediate (+)-B[_a_]PDE in the lung. This either reacts with dGuo in DNA to form the (+)-B[_a_]PDE-dGuo adduct and initiates mutagenesis or is detoxified by GST to the (−)-B[_a_]PDE-GSH-adduct. TCDD induces P450s 1A1 and 1B1 through the AhR to increase the activation of B[_a_]P. It is proposed that there is another AhR-mediated interaction, which results in the increased expression of GSTs that can detoxify (+)-B[_a_]PDE with high enantioselectivity. A balance between the activation and detoxification pathways would then determine the amount of DNA damage that occurs.
References
- Baird W. M.; Hooven L. A.; Mahadevan B. (2005) Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ. Mol. Mutagen. 45, 2−3106–114. - PubMed
- Hecht S. S. (2006) Cigarette smoking: cancer risks, carcinogens, and mechanisms. Langenbecks Arch. Surg. 391, 6603–613. - PubMed
- Ruan Q.; Gelhaus S. L.; Penning T. M.; Harvey R. G.; Blair I. A. (2007) Aldo-keto reductase- and cytochrome P450-dependent formation of benzo[_a_]pyrene-derived DNA-adducts in human bronchoalveolar cells. Chem. Res. Toxicol. 20, 3424–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 ES015857/ES/NIEHS NIH HHS/United States
- R01ES015857/ES/NIEHS NIH HHS/United States
- P30 ES013508/ES/NIEHS NIH HHS/United States
- 5F32ES016683/ES/NIEHS NIH HHS/United States
- P30ES013508/ES/NIEHS NIH HHS/United States
- R01 CA130038/CA/NCI NIH HHS/United States
- R01CA130038/CA/NCI NIH HHS/United States
- F32 ES016683/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources