Pre-clinical and clinical significance of heparanase in Ewing's sarcoma - PubMed (original) (raw)
. 2011 Sep;15(9):1857-64.
doi: 10.1111/j.1582-4934.2010.01190.x.
Myriam Weyl Ben-Arush, Josephine Issakov, Isaac Meller, Inna Naroditsky, Monica Tortoreto, Giuliana Cassinelli, Cinzia Lanzi, Claudio Pisano, Neta Ilan, Israel Vlodavsky, Franco Zunino
Affiliations
- PMID: 21029368
- PMCID: PMC3056168
- DOI: 10.1111/j.1582-4934.2010.01190.x
Pre-clinical and clinical significance of heparanase in Ewing's sarcoma
Itay Shafat et al. J Cell Mol Med. 2011 Sep.
Abstract
Heparanase is an endoglycosidase that specifically cleaves heparan sulphate side chains of heparan sulphate proteoglycans, activity that is strongly implicated in cell migration and invasion associated with tumour metastasis, angiogenesis and inflammation. Heparanase up-regulation was documented in an increasing number of human carcinomas, correlating with reduced post-operative survival rate and enhanced tumour angiogenesis. Expression and significance of heparanase in human sarcomas has not been so far reported. Here, we applied the Ewing's sarcoma cell line TC71 and demonstrated a potent inhibition of cell invasion in vitro and tumour xenograft growth in vivo upon treatment with a specific inhibitor of heparanase enzymatic activity (compound SST0001, non-anticoagulant N-acetylated, glycol split heparin). Next, we examined heparanase expression and cellular localization by immunostaining of a cohort of 69 patients diagnosed with Ewing's sarcoma. Heparanase staining was noted in all patients. Notably, heparanase staining intensity correlated with increased tumour size (P = 0.04) and with patients' age (P = 0.03), two prognostic factors associated with a worse outcome. Our study indicates that heparanase expression is induced in Ewing's sarcoma and associates with poor prognosis. Moreover, it encourages the inclusion of heparanase inhibitors (i.e. SST0001) in newly developed therapeutic modalities directed against Ewing's sarcoma and likely other malignancies.
© 2011 The Authors Journal compilation © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures
Fig 1
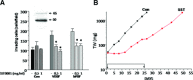
(A) Effect of SST0001 on the invasive ability of TC71 cells stimulated by exogenous VEGF or bFGF. Cells, pre-treated with the indicated concentration of SST0001 in complete medium for 24 hrs, were subjected to Matrigel invasion assay in serum-free medium with or without VEGF or bFGF (50 ng/ml). The number of invading cells per field is reported. Columns represent mean values ± S.D. of two replicates. One representative experiment is shown. *P < 0.0001. Inset: Expression of heparanase in TC71 cells. Whole cell lysate was subjected to immunoblotting with the anti-HPA1 antibody. Arrows indicate the latent (65 kD) and active (50 kD) heparanase forms. (B) Antitumor activity of SST0001 against TC71 tumour xenograft. SST0001 was dissolved in saline and administered by two daily s.c. injections of 60 mg/kg for 23 consecutive days. (▪), control; (•), SST0001.
Fig 2
Immunohistochemical staining of heparanase in Ewing’s sarcoma specimens. Formalin-fixed, paraffin-embedded 5 μm sections were subjected to immunostaining, applying anti-heparanase polyclonal antibody (Ab 733), as described under ‘Materials and methods’. Shown are representative photomicrographs of heparanase-positive specimens categorized as weak (+1, A, B) or strong (+2, C, D) intensity. Nuclear localization of heparanase is shown in (E). Specimens that were similarly stained with pre-immune serum, or applying the above procedure but lacking the primary antibody, yielded no detectable staining (F). Original magnification: (A, C) 100×; (B, D–F) 400×.
Similar articles
- Antitumor efficacy of the heparan sulfate mimic roneparstat (SST0001) against sarcoma models involves multi-target inhibition of receptor tyrosine kinases.
Cassinelli G, Favini E, Dal Bo L, Tortoreto M, De Maglie M, Dagrada G, Pilotti S, Zunino F, Zaffaroni N, Lanzi C. Cassinelli G, et al. Oncotarget. 2016 Jul 26;7(30):47848-47863. doi: 10.18632/oncotarget.10292. Oncotarget. 2016. PMID: 27374103 Free PMC article. - Antitumor efficacy of the heparanase inhibitor SST0001 alone and in combination with antiangiogenic agents in the treatment of human pediatric sarcoma models.
Cassinelli G, Lanzi C, Tortoreto M, Cominetti D, Petrangolini G, Favini E, Zaffaroni N, Pisano C, Penco S, Vlodavsky I, Zunino F. Cassinelli G, et al. Biochem Pharmacol. 2013 May 15;85(10):1424-32. doi: 10.1016/j.bcp.2013.02.023. Epub 2013 Mar 1. Biochem Pharmacol. 2013. PMID: 23466421 - Targeting Lyn inhibits tumor growth and metastasis in Ewing's sarcoma.
Guan H, Zhou Z, Gallick GE, Jia SF, Morales J, Sood AK, Corey SJ, Kleinerman ES. Guan H, et al. Mol Cancer Ther. 2008 Jul;7(7):1807-16. doi: 10.1158/1535-7163.MCT-08-0058. Mol Cancer Ther. 2008. PMID: 18644993 Free PMC article. - Heparanase: A Potential Therapeutic Target in Sarcomas.
Cassinelli G, Lanzi C. Cassinelli G, et al. Adv Exp Med Biol. 2020;1221:405-431. doi: 10.1007/978-3-030-34521-1_15. Adv Exp Med Biol. 2020. PMID: 32274719 Review. - The heparanase/heparan sulfate proteoglycan axis: A potential new therapeutic target in sarcomas.
Cassinelli G, Zaffaroni N, Lanzi C. Cassinelli G, et al. Cancer Lett. 2016 Nov 28;382(2):245-254. doi: 10.1016/j.canlet.2016.09.004. Epub 2016 Sep 22. Cancer Lett. 2016. PMID: 27666777 Review.
Cited by
- Involvement of heparanase in atherosclerosis and other vessel wall pathologies.
Vlodavsky I, Blich M, Li JP, Sanderson RD, Ilan N. Vlodavsky I, et al. Matrix Biol. 2013 Jun 24;32(5):241-51. doi: 10.1016/j.matbio.2013.03.002. Epub 2013 Mar 13. Matrix Biol. 2013. PMID: 23499530 Free PMC article. Review. - Heparanase: From basic research to therapeutic applications in cancer and inflammation.
Vlodavsky I, Singh P, Boyango I, Gutter-Kapon L, Elkin M, Sanderson RD, Ilan N. Vlodavsky I, et al. Drug Resist Updat. 2016 Nov;29:54-75. doi: 10.1016/j.drup.2016.10.001. Epub 2016 Oct 6. Drug Resist Updat. 2016. PMID: 27912844 Free PMC article. Review. - Heparanase in inflammation and inflammation-associated cancer.
Meirovitz A, Goldberg R, Binder A, Rubinstein AM, Hermano E, Elkin M. Meirovitz A, et al. FEBS J. 2013 May;280(10):2307-19. doi: 10.1111/febs.12184. Epub 2013 Mar 4. FEBS J. 2013. PMID: 23398975 Free PMC article. Review. - The prognostic significance of heparanase expression in metastatic melanoma.
Vornicova O, Boyango I, Feld S, Naroditsky I, Kazarin O, Zohar Y, Tiram Y, Ilan N, Ben-Izhak O, Vlodavsky I, Bar-Sela G. Vornicova O, et al. Oncotarget. 2016 Nov 15;7(46):74678-74685. doi: 10.18632/oncotarget.12492. Oncotarget. 2016. PMID: 27732945 Free PMC article. - Antitumor efficacy of the heparan sulfate mimic roneparstat (SST0001) against sarcoma models involves multi-target inhibition of receptor tyrosine kinases.
Cassinelli G, Favini E, Dal Bo L, Tortoreto M, De Maglie M, Dagrada G, Pilotti S, Zunino F, Zaffaroni N, Lanzi C. Cassinelli G, et al. Oncotarget. 2016 Jul 26;7(30):47848-47863. doi: 10.18632/oncotarget.10292. Oncotarget. 2016. PMID: 27374103 Free PMC article.
References
- Esiashvili N, Goodman M, Marcus RB., Jr Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: surveillance epidemiology and end results data. J Pediatr Hematol Oncol. 2008;30:425–30. - PubMed
- Leavey PJ, Collier AB. Ewing sarcoma: prognostic criteria, outcomes and future treatment. Expert Rev Anticancer Ther. 2008;8:617–24. - PubMed
- Riggi N, Stamenkovic I. The biology of Ewing sarcoma. Cancer Lett. 2007;254:1–10. - PubMed
- Paulussen M, Bielack S, Jurgens H, et al. Ewing’s sarcoma of the bone: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20:140–2. - PubMed
- Bernstein M, Kovar H, Paulussen M, et al. Ewing’s sarcoma family of tumors: current management. Oncologist. 2006;11:503–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources