The importin β binding domain as a master regulator of nucleocytoplasmic transport - PubMed (original) (raw)
Review
The importin β binding domain as a master regulator of nucleocytoplasmic transport
Kaylen Lott et al. Biochim Biophys Acta. 2011 Sep.
Abstract
Specific and efficient recognition of import cargoes is essential to ensure nucleocytoplasmic transport. To this end, the prototypical karyopherin importin β associates with import cargoes directly or, more commonly, through import adaptors, such as importin α and snurportin. Adaptor proteins bind the nuclear localization sequence (NLS) of import cargoes while recruiting importin β via an N-terminal importin β binding (IBB) domain. The use of adaptors greatly expands and amplifies the repertoire of cellular cargoes that importin β can efficiently import into the cell nucleus and allows for fine regulation of nuclear import. Accordingly, the IBB domain is a dedicated NLS, unique to adaptor proteins that functions as a molecular liaison between importin β and import cargoes. This review provides an overview of the molecular role played by the IBB domain in orchestrating nucleocytoplasmic transport. Recent work has determined that the IBB domain has specialized functions at every step of the import and export pathway. Unexpectedly, this stretch of ~40 amino acids plays an essential role in regulating processes such as formation of the import complex, docking and translocation through the nuclear pore complex (NPC), release of import cargoes into the cell nucleus and finally recycling of import adaptors and importin β into the cytoplasm. Thus, the IBB domain is a master regulator of nucleocytoplasmic transport, whose complex molecular function is only recently beginning to emerge. This article is part of a Special Issue entitled: Regulation of Signaling and Cellular Fate through Modulation of Nuclear Protein Import.
2010 Elsevier B.V. All rights reserved.
Figures
Fig. 1. Diversification of NLSs three-dimensional structure and amino acid sequence
All structures are taken from the crystallographic complexes of the NLS bound to their respective receptor/adaptor. In the amino acid sequence, basic amino acids are colored in blue. (A) Classical monopartite and bipartite NLS found in SV40 T-large antigen (pdb 1EJL) and nucleoplasmin (pdb 1EJY) (top and bottom, respectively) that bind importin α. (B) Extended non-classical NLSs recognized directly by β-karyopherins. In the left panel is the PTHrP-NLS (res. 67–94) (pdb 1M5N) that binds importin β N-terminal HEAT repeats 1–11 [13]. In the right panel is the M9-NLS of hnRNP A1, which binds transportin (pdb 2H4M) [12]. (C) Ribbon diagram of importin α IBB-domain (pdb 1QGK). This ∼40 amino acid domain contains an N-terminal 310 helix connected by a loop of variable length to a long C-terminal α-helix.
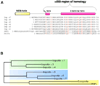
Fig. 2. Evolution and conservation of IBB-domains in adaptor proteins
(A) Sequence alignment of IBB-domains found in six human isoforms of importin α (αl, α3, α4, α5, α6, α7), human snurportin (SNP1), and human XRIPα. A schematic representation of the IBB-secondary structure is drawn above the sequence alignment. In the alignment, highly conserved basic residues are colored in red. The underlined sequences refer to the IBB-domains that were crystallized in complex with importin β, namely importin αl IBB-domain (res. 11–54) [9] and snurportin IBB-domain (res. 25–65) [10]. Notice that the N-terminal extension comprising the NES [56] is conserved only in sIBB, and in other snurportin homologues [10], but not in αIBBs and xXRIPα. (B) Phylogenetic tree of human IBB-domain sequences shown in panel (A). In the tree, the branch lengths are proportional to the predicted evolutionary time between sequences. Three subfamilies of IBBs (shaded in green, blue and yellow) are identified. Both sequence alignment and phylogenetic tree were generated using the program ClustalW [118].
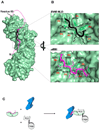
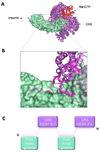
Fig. 3. The autoinhibited conformation of importin α IBB-domain
(A) Surface representation of the mammalian importin α (in green) with the αIBB-domain (in magenta) in an autoinhibited conformation (pdb 1IAL). The residues that connect the autoinhibitory IBB-sequence with the ARM repeat core, as well as those amino acids N-terminal of the bound peptide were not seen in the crystal structure and therefore are modeled as a magenta dashed line. The structure of the SV40 NLS (in black) is also docked to importin α. The NLS and the IBB occupy the same position on importin α. (B) Blowup view of the binding site for NLS (top) and IBB (bottom) in importin α. Key interactions between the NLS/IBB and importin α at sites P1-P6 are shown. Importin α Trp and Asn residues contacting basic NLS/IBB side chains are shown as green sticks. (C) Cartoon schematic depicting how the IBB functions to regulate formation of the trimeric import complex of importin β/α/NLS when all three factors are present.
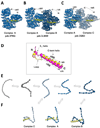
Fig. 4. Structure and recognition of IBB-domains by importin β
Structural views of importin β bound to the sIBB (res 25–65) in three distinct crystal forms. (A) 2.35Å resolution structure (pdb 2P8Q) of importin β bound to sIBB (res. 25–65) containing one complex in the asymmetric unit. This structure represents the strained conformation of the protein. (B) 3.15Å resolution structure (pdb 3LWW) with two importin β/sIBB complexes (indicated as A–B) in the same asymmetric unit. Complex A is identical to the high resolution structure in panel (A). In contrast, complex B presents an ultra-strained conformation of importin β (C) 3.2Å resolution structure (pdb 2Q5D) with two importin β/sIBB (res. 25–65) complexes (indicated as A–C) in the same asymmetric unit. Complex A is equivalent to importin β/sIBB complex A in panel (A) and (B), while complex C has a dramatically less strained conformation of importin β. All structures in (A–C) are aligned using importin β in complex A as reference. In all complexes, the sIBB is colored in yellow and the distance between N- and C-terminal HEAT repeats of importin β is indicated. (D) Ribbon diagram of sIBB superimposed onto the αIBB domain (in yellow and magenta, respectively). Both IBBs were crystallized in complex with full length human importin β (pdb 2P8Q and 1QGK, respectively). The sIBB α-helix is ∼5 Å shorter than the αIBB C-terminal α-helix due to a three amino acid gap in its amino acid sequence between residues 46–47 (Fig. 2A). (E) Population selection model proposed for the recognition of sIBB by importin β. Putative conformers of importin β are shown as beads-on-string, where each HEAT repeat is represented by a sphere. This view emphasizes the different degree of intramolecular opening in different importin β conformers. Grey molecules are conformations of importin β not observed crystallographically, but likely seen by small angle X-ray scattering [72]. (F) Stabilization of different conformers by the sIBB-domain is thought to populate importin β conformers, which can be crystallized and hence trapped in a crystal lattice [75].
Fig. 5. Role of IBB-domain in translocation of import complexes through the NPC
Structure of the importin β/sIBB complex (colored in blue and yellow, respectively) at the central channel of the NPC, which is formed primarily by Nup62 complex [119]. This schematic is not to scale: the structure of importin β is greatly magnified with respect to the NPC central channel. Key FG-nucleoporins are indicated respective to their location in the NPC. In the blowup is cartoon of a region of the importin β HEAT repeats bound to FG-motifs. HEAT repeats are represented as circles, with the outer A helices in dark blue, and the concave B helices as light blue. B helices are responsible for binding to the IBB-domain while the convex surface interacts with the nucleoporins. Phenylalanine side chains emanating from FG-nups are modeled interacting with the groove in between A helices of importin β.
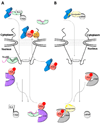
Fig. 6. Role of IBB-domain in recycling of adaptors and importin β to the cytoplasm
(A) Cartoon diagram of the importin β/α/NLS-cargo complex (in blue, green, and grey, respectively) assembled in the cytoplasm. Upon nuclear entry RanGTP (in red) displaces importin β, and the nucleoplasmic nucleoporins Nup50 (black line) binds importin α NLS-groove and displaces the NLS-cargo. The export complex of CAS and RanGTP (shown in purple and red, respectively) displaces Nup50 from importin α and with the αIBB locked in an autoinhibited position an export-competent forms to be recycled to the cytoplasm. (B) An analogous scenario for the importin β/snurportin/snRNP import complex (in blue, yellow and grey, respectively). Upon nuclear entry the N-terminal seventeen amino acids of the sIBB binds to the export factor CRM1 (shown in silver). Colored in dark yellow is the remainder of the sIBB not involved in interactions with CRM1, shown as two surface exposed helices separated by a kinked loop.
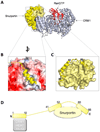
Fig. 7. Structure of the αIBB in the importin α/CAS/RanGTP export complex
(A) Structure of the trimeric importin α export complex (pdb 1WA5) [78], which consists of Cse1p (yeast homologue of CAS), RanGTP and Kap60p (yeast homologue of importin α), in purple, red and green, respectively. The IBB-domain is colored in magenta. (B) Zoom in view of αIBB interaction with Cse1p and the NLS binding grooves of importin α. IBB residues 20–31 were not observed in the crystal structure and are modeled as a dashed line. (C) Schematic representation of the αIBB emphasizing the complex binding interactions of IBB-domain (magenta) with Cse1p (purple) and the NLS binding sites on importin α (green).
Fig. 8. Structure of the sIBB in the snurportin/CRM1/RanGTP export complex
(A) Structure of the snurportin export complex formed by CRM1 (silver ribbon), RanGTP (red ribbon) and snurportin (shown as a light yellow surface) (pdb 3GJX) [104], with the sIBB highlighted as a yellow cartoon. (B) The N-terminus of the sIBB (shown as a yellow helix) occupies a hydrophobic cleft formed by CRM1 HEAT repeats 11–12. In the diagram, the acidic surface of CRM1 electrostatic potential map is colored in red, while the NES binding groove, which is more hydrophobic, is in white. (C) Magnification of sIBB residues 41–66 bound as an interrupted helix to the main body of snurportin. The sIBB is shown as a yellow ribbon, while snurportin main body is shown as a yellow surface. The surface exposed kink between sIBB helix 41–52 and 60–66 is a possible target for importin β. (D) Schematic representation of the sIBB-domain emphasizing its extended interactions with CRM1 (silver) and snurportin (yellow).
Similar articles
- Nuclear Respiratory Factor 2β (NRF-2β) recruits NRF-2α to the nucleus by binding to importin-α:β via an unusual monopartite-type nuclear localization signal.
Hayashi R, Takeuchi N, Ueda T. Hayashi R, et al. J Mol Biol. 2013 Sep 23;425(18):3536-48. doi: 10.1016/j.jmb.2013.07.007. Epub 2013 Jul 13. J Mol Biol. 2013. PMID: 23856623 - Molecular basis for specificity of nuclear import and prediction of nuclear localization.
Marfori M, Mynott A, Ellis JJ, Mehdi AM, Saunders NF, Curmi PM, Forwood JK, Bodén M, Kobe B. Marfori M, et al. Biochim Biophys Acta. 2011 Sep;1813(9):1562-77. doi: 10.1016/j.bbamcr.2010.10.013. Epub 2010 Oct 25. Biochim Biophys Acta. 2011. PMID: 20977914 Review. - Nuclear import by karyopherin-βs: recognition and inhibition.
Chook YM, Süel KE. Chook YM, et al. Biochim Biophys Acta. 2011 Sep;1813(9):1593-606. doi: 10.1016/j.bbamcr.2010.10.014. Epub 2010 Oct 26. Biochim Biophys Acta. 2011. PMID: 21029754 Free PMC article. Review. - Molecular basis for the recognition of snurportin 1 by importin beta.
Mitrousis G, Olia AS, Walker-Kopp N, Cingolani G. Mitrousis G, et al. J Biol Chem. 2008 Mar 21;283(12):7877-84. doi: 10.1074/jbc.M709093200. Epub 2008 Jan 9. J Biol Chem. 2008. PMID: 18187419 - The importin beta binding domain modulates the avidity of importin beta for the nuclear pore complex.
Lott K, Bhardwaj A, Mitrousis G, Pante N, Cingolani G. Lott K, et al. J Biol Chem. 2010 Apr 30;285(18):13769-80. doi: 10.1074/jbc.M109.095760. Epub 2010 Mar 1. J Biol Chem. 2010. PMID: 20197273 Free PMC article.
Cited by
- Karyopherins in the Remodeling of Extracellular Matrix: Implications in Tendon Injury.
Diaz C, Thankam FG, Agrawal DK. Diaz C, et al. J Orthop Sports Med. 2023;5(3):357-374. doi: 10.26502/josm.511500122. Epub 2023 Sep 14. J Orthop Sports Med. 2023. PMID: 37829147 Free PMC article. - Evidence for an evolutionary relationship between the large adaptor nucleoporin Nup192 and karyopherins.
Stuwe T, Lin DH, Collins LN, Hurt E, Hoelz A. Stuwe T, et al. Proc Natl Acad Sci U S A. 2014 Feb 18;111(7):2530-5. doi: 10.1073/pnas.1311081111. Epub 2014 Feb 6. Proc Natl Acad Sci U S A. 2014. PMID: 24505056 Free PMC article. - Insights from the Molecular Modelling and Docking Analysis of AIF-NLS complex to infer Nuclear Translocation of the Protein.
Srivaths A, Ramanathan S, Sakthivel S, Habeeb S. Srivaths A, et al. Bioinformation. 2018 Mar 31;14(3):132-139. doi: 10.6026/97320630014132. eCollection 2018. Bioinformation. 2018. PMID: 29785072 Free PMC article. - The Influenza A Virus Replication Cycle: A Comprehensive Review.
Carter T, Iqbal M. Carter T, et al. Viruses. 2024 Feb 19;16(2):316. doi: 10.3390/v16020316. Viruses. 2024. PMID: 38400091 Free PMC article. Review. - Diversification of importin-α isoforms in cellular trafficking and disease states.
Pumroy RA, Cingolani G. Pumroy RA, et al. Biochem J. 2015 Feb 15;466(1):13-28. doi: 10.1042/BJ20141186. Biochem J. 2015. PMID: 25656054 Free PMC article. Review.
References
- Kalderon D, Richardson WD, Markham AF, Smith AE. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984;311:33–38. - PubMed
- Kalderon D, Roberts BL, Richardson WD, Smith AE. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. - PubMed
- Robbins J, Dilworth SM, Laskey RA, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991;64:615–623. - PubMed
- Jans DA, Xiao CY, Lam MH. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays. 2000;22:532–544. - PubMed
- Sessler RJ, Noy N. A ligand-activated nuclear localization signal in cellular retinoic acid binding protein-II. Mol Cell. 2005;18:343–353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources