Site-mapping of in vitro S-nitrosation in cardiac mitochondria: implications for cardioprotection - PubMed (original) (raw)
Site-mapping of in vitro S-nitrosation in cardiac mitochondria: implications for cardioprotection
Christopher I Murray et al. Mol Cell Proteomics. 2011 Mar.
Abstract
S-nitrosation (SNO) of mitochondrial protein cysteines can be cardioprotective. Several targets have been implicated, yet the scope and identification of specific residues has not been fully assessed. To address this, a comprehensive assessment of mitochondrial SNO-modifiable cysteines was performed to determine nitric oxide (NO) susceptible pathways and identify novel mechanisms of oxidative cardioprotection. The biotin switch assay and mass spectrometry were used on rat cardiac mitochondrial lysates treated with the nitric oxide donor, S-nitrosoglutathione, and controls (n=3) to map 83 SNO-modified cysteine residues on 60 proteins. Of these, three sites have been reported, 30 sites are new to 21 proteins previously known to be S-nitrosated but which lacked site-specific information and 50 sites were found on 39 proteins not previously implicated in SNO pathways. The SNO-modifications occurred in only a subset of available cysteines, indicating a specific targeted effect. Functional annotation and site-specificity analysis revealed a twofold greater nitric oxide-susceptibility for proteins involved in transport; including regulators of mitochondrial permeability transition suggesting SNO-regulation and a possible protective mechanism. Additionally, we identified many novel SNO-modified proteins with cardioprotective potential involved in the electron transport chain, tricarboxylic acid cycle, oxidative stress defense, fatty acid and amino acid metabolism. These findings suggest that SNO-modification may represent a novel mechanism for the regulation of oxidative phosphorylation and/or cell death. S-nitrosation of mitochondrial permeability transition-associated proteins represents an intriguing potential link to cardioprotection.
Figures
Fig. 1.
Detection and site mapping of cardiac mitochondrial SNO-modifications. A, Biotin switch assay schema outlining the blocking, reducing and biotin labeling steps as well as capture of intact proteins or digested peptides for LC/MS/MS (19, 21). B, Representative silver stained gel of SNO-modified proteins captured from 250 μg of rat cardiac mitochondria treated with 100 μmol/L GSNO, 100 μmol/L GSH, 5 mmol/L DTT or untreated vehicle with and without NEM blocking and subjected to the biotin switch assay (n = 3). A description of each of the control treatments can be found in the text.
Fig. 2.
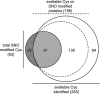
Comparison of the SNO sites to all available cysteines in cardiac mitochondria reveals site-specificity. Identified sites are summarized in a Venn diagram. SNO-modified sites (dark gray) compared with all available cysteines (white). A total of 285 available cysteines were identified, 61 of those corresponded with SNO-modified sites indicating specificity in NO-modification (light gray). Among only the SNO-modified proteins, a total of 196 available sites were found (dashed line), 135 of which were found to be unmodified by NO. Also represented are the 22 SNO-sites not detected in the available cysteine data set. This absence is likely because of the increased complexity of the unblocked samples in the MS analysis.
Fig. 3.
Frequency of amino acids surrounding S-nitrosated cysteines and comparison of secondary structural elements adjacent to SNO-modified and unmodified cysteine residues. A, Analysis of the flanking sequences of 50 S-nitrosated cysteines, randomly chosen from the total of 84 detected modified sites, did not reveal any obvious pattern. However, comparison with a recent analysis on _S_-nitrosated targets in prostate epithelial cells (52) suggest a preference for aliphatic residues at positions −10 to −7; hydrophobic residues at positions −4 and −1, and two glycines at positions +2 and +3 relative to the modified cysteines. Hydrophobic residues are represented in black; charged residues in red and blue. Other residues are shown in either magenta or green. B, Comparison of the predicted secondary structures flanking nitrosated and unmodified sites might suggest a preference for reduced entropy as indicated by the elevated frequency of α-helix (30% versus 15%) and reduced frequency of coil (44% versus 53%). The modified cysteines appear to have a higher tendency to be buried (96% versus 80%), and show on average an ∼1.9-fold smaller predicted surface accessibility as compared with the set of unmodified cysteines. α: alpha-helix; β: beta sheet; c: coil.
Fig. 4.
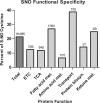
Summary of cysteine SNO-susceptibility by function. Summary of the percent of SNO modifications compared with the available cysteines for the major functional pathways in cardiac mitochondria. The specific values for each functional group are listed above each bar (number of SNO-cysteines/number of available cysteines).
Similar articles
- Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury.
Nadtochiy SM, Burwell LS, Brookes PS. Nadtochiy SM, et al. J Mol Cell Cardiol. 2007 Apr;42(4):812-25. doi: 10.1016/j.yjmcc.2007.01.010. Epub 2007 Jan 31. J Mol Cell Cardiol. 2007. PMID: 17350035 Free PMC article. - Identification of novel S-nitrosation sites in soluble guanylyl cyclase, the nitric oxide receptor.
Beuve A, Wu C, Cui C, Liu T, Jain MR, Huang C, Yan L, Kholodovych V, Li H. Beuve A, et al. J Proteomics. 2016 Apr 14;138:40-7. doi: 10.1016/j.jprot.2016.02.009. Epub 2016 Feb 18. J Proteomics. 2016. PMID: 26917471 Free PMC article. - Regulation of mitochondrial processes by protein S-nitrosylation.
Piantadosi CA. Piantadosi CA. Biochim Biophys Acta. 2012 Jun;1820(6):712-21. doi: 10.1016/j.bbagen.2011.03.008. Epub 2011 Mar 21. Biochim Biophys Acta. 2012. PMID: 21397666 Free PMC article. Review. - Identification of S-nitrosated mitochondrial proteins by S-nitrosothiol difference in gel electrophoresis (SNO-DIGE): implications for the regulation of mitochondrial function by reversible S-nitrosation.
Chouchani ET, Hurd TR, Nadtochiy SM, Brookes PS, Fearnley IM, Lilley KS, Smith RA, Murphy MP. Chouchani ET, et al. Biochem J. 2010 Aug 15;430(1):49-59. doi: 10.1042/BJ20100633. Biochem J. 2010. PMID: 20533907 Free PMC article. - Protein _S_-Nitrosation: Biochemistry, Identification, Molecular Mechanisms, and Therapeutic Applications.
Ye H, Wu J, Liang Z, Zhang Y, Huang Z. Ye H, et al. J Med Chem. 2022 Apr 28;65(8):5902-5925. doi: 10.1021/acs.jmedchem.1c02194. Epub 2022 Apr 12. J Med Chem. 2022. PMID: 35412827 Review.
Cited by
- Proteomics in heart failure: top-down or bottom-up?
Gregorich ZR, Chang YH, Ge Y. Gregorich ZR, et al. Pflugers Arch. 2014 Jun;466(6):1199-209. doi: 10.1007/s00424-014-1471-9. Epub 2014 Mar 13. Pflugers Arch. 2014. PMID: 24619480 Free PMC article. Review. - ROLE OF THIOLS IN OXIDATIVE STRESS.
Baba SP, Bhatnagar A. Baba SP, et al. Curr Opin Toxicol. 2018 Feb;7:133-139. doi: 10.1016/j.cotox.2018.03.005. Epub 2018 Mar 21. Curr Opin Toxicol. 2018. PMID: 30338308 Free PMC article. - S-Nitrosoglutathione Reductase Is Essential for Protecting the Female Heart From Ischemia-Reperfusion Injury.
Casin KM, Fallica J, Mackowski N, Veenema RJ, Chan A, St Paul A, Zhu G, Bedja D, Biswal S, Kohr MJ. Casin KM, et al. Circ Res. 2018 Nov 9;123(11):1232-1243. doi: 10.1161/CIRCRESAHA.118.313956. Circ Res. 2018. PMID: 30571462 Free PMC article. - Protein _S_-Nitrosylation Controls Glycogen Synthase Kinase 3β Function Independent of Its Phosphorylation State.
Wang SB, Venkatraman V, Crowgey EL, Liu T, Fu Z, Holewinski R, Ranek M, Kass DA, O'Rourke B, Van Eyk JE. Wang SB, et al. Circ Res. 2018 May 25;122(11):1517-1531. doi: 10.1161/CIRCRESAHA.118.312789. Epub 2018 Mar 21. Circ Res. 2018. PMID: 29563102 Free PMC article. - Redox regulation of mitochondrial function.
Handy DE, Loscalzo J. Handy DE, et al. Antioxid Redox Signal. 2012 Jun 1;16(11):1323-67. doi: 10.1089/ars.2011.4123. Epub 2012 Feb 3. Antioxid Redox Signal. 2012. PMID: 22146081 Free PMC article. Review.
References
- Jones S. P., Bolli R. (2006) The ubiquitous role of nitric oxide in cardioprotection. J. Mol. Cell Cardiol. 1, 16–23 - PubMed
- Hess D. T., Matsumoto A., Kim S. O., Marshall H. E., Stamler J. S. (2005) Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2, 150–166 - PubMed
- Borutaite V., Brown G. C. (2006) S-nitrosothiol inhibition of mitochondrial complex I causes a reversible increase in mitochondrial hydrogen peroxide production. Biochim. Biophys. Acta. 5–6, 562–566 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL077180/HL/NHLBI NIH HHS/United States
- P01 HL081427/HL/NHLBI NIH HHS/United States
- P01-HL081427/HL/NHLBI NIH HHS/United States
- P01-HL077180/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases