Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium - PubMed (original) (raw)
Randomized Controlled Trial
. 2010 Dec 1;185(11):7107-14.
doi: 10.4049/jimmunol.1002756. Epub 2010 Oct 29.
Affiliations
- PMID: 21037100
- PMCID: PMC3075978
- DOI: 10.4049/jimmunol.1002756
Randomized Controlled Trial
Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium
Cornelia L Trimble et al. J Immunol. 2010.
Abstract
High-grade cervical dysplasia caused by human papillomavirus (HPV) type 16 is a lesion that should be susceptible to an HPV-specific immune response; disease initiation and persistence is predicated on expression of two viral Ags, E6 and E7. In immune-competent subjects, at least 25% of HPV16(+) high-grade cervical dysplasia lesions undergo complete regression. However, in the peripheral blood, naturally occurring IFN-γ T cell responses to HPV E6 and E7 are weak, requiring ex vivo sensitization to detect, and are not sufficiently sensitive to predict regression. In this study, we present immunologic data directly assessing cervical lymphocytes from this cohort. We found that nearly all cervical tissue T cells express the mucosal homing receptor, α(4)β(7) surface integrin. T cells isolated from dysplastic mucosa were skewed toward a central memory phenotype compared with normal mucosal resident T cells, and dysplastic lesions expressed transcripts for CCL19 and CCL21, raising the possibility that the tissue itself sustains a response that is not detectable in the blood. Moreover, lesion regression in the study window could retrospectively be predicted at study entry by the ability of CD8(+) T cells to gain access to lesional epithelium. Vascular endothelial expression of mucosal addressin cell adhesion molecule-1, the ligand that supports entry of α(4)β(7)(+) T cells into tissues, colocalized tightly with the distribution of CD8 T cells and was not expressed in persistent dysplastic epithelium. These findings suggest that dysregulated expression of vascular adhesion molecules plays a role in immune evasion very early in the course of HPV disease.
Conflict of interest statement
Disclosures
The authors have no financial conflicts of interest.
Figures
FIGURE 1
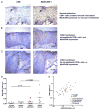
CD8+ infiltrates localize to dysplastic mucosal. A, Representative CD8 immunohistochemical staining of persistent CIN2/3 adjacent to normal mucosa. CD8+ cells localize to dysplastic stroma compared with immediately adjacent normal tissue. ROIs are demarcated as indicated to illustrate the method used to quantitate the intensity of immunostaining (original magnification ×100). B, Representative section depicting CD8+ cells in normal cervical squamous mucosa distributed along the basal epithelial layer and clustered around a vessel extending into the squamous compartment (immunohistochemical staining, original magnification ×400). C, Representative section depicting CD8+ cell penetration into lesional epithelium in t0 biopsy specimen, lesion regressor (immunohistochemical staining, original magnification ×400). D, Representative example of lack of CD8+ cells in lesional epithelium at t0, nonregressor (immunohistochemical staining, original magnification ×400). E, Quantification of CD8+ infiltrates in cervical mucosa in normal (control) mucosa (n = 15) and from lesion sites at t0 in lesions that regressed (n = 7) and lesions that did not regress (n = 20). At least 3 and up to 10 fields from each section were counted to define the density of infiltrates. F, Quantification of stromal and epithelial CD8+ in nonregressing lesions (n = 20), at study entry (t0), and at the time of excision (twk15).
FIGURE 2
Dysplastic mucosa recruits activated memory T cells. A, Representative flow cytometry analysis of the percentage of CD3+CD45RO+ and CD3+CD45RA+ T cells in normal and CIN2/3 cervix. B, Quantification of CD45RA+ and CD45RO+CD3+ T cells in normal (n = 6) and CIN2/3 cervix (n = 4). C, Representative flow cytometry analysis of the percentage of CD3+ T cells expressing activation markers CD25 and CD69 in normal and CIN2/3 cervixes. D, Quantification of CD3+CD45RO+ T cells expressing CD25 and CD69 in normal (n = 3) and CIN2/3 (n = 3) cervixes. E, Representative flow cytometry analysis of CD3+CD45RO+ T cells expressing surface phenotype of effector memory T cells (CCR7−CD62L−) versus TCM (CCR7+CD62L+) in normal and CIN2/3 cervixes. F, Quantification of effector memory T cells and TCM in normal (n = 6) and CIN2/3 (n = 6) cervixes. G, Representative histologic sections of tonsil (left panels) and CIN2/3 cervix (right panels), immunostained for CD62L (top panels) and PNAd (bottom panels) demonstrate colocalization of cells expressing CD62L, and endothelial vasculature expressing PNAd (original magnification ×200). H, CCL19 and CCL21 mRNA transcripts relative to GADPH were quantified by quantitative real-time RT-PCR in normal (n = 3) and CIN2/3 (n = 4) flash-frozen cervical tissue samples.
FIGURE 3
Colpograph of CIN2/3: acetowhite epithelium (dashed red line) with characteristic patterns of neovascularization, including punctation, and mosaicism (bold arrow). Original magnification ×10.
FIGURE 4
Cervical tissue T cells express the α4β7 surface integrin. A, Representative flow cytometry surface phenotyping analyses of T cells isolated from normal cervixes (left panels) and CIN2/3 cervixes (right panels). B, Quantification of the percent of cervical CD3+ T cells expressing α4β7, CCR4, and CLA in normal (n = 5) and CIN2/3 (n = 4) cervixes.
FIGURE 5
Tissue expression of MAdCAM colocalizes with CD8+ infiltrates in CIN2/3 epithelium. A, Representative serial tissue sections of normal cervical epithelium, immunostained for CD8 (left panel) and MAdCAM-1 (right panel). Arrows identify perivascular CD8+ cells (left panel) and MAdCAM-1 expression in corresponding vessels (right panel) (original magnification ×400). B, Representative CIN2/3 epithelium containing CD8+ cell infiltrates (left panel), with MAdCAM-1 expression in a serial section, same field (right panel) (original magnification ×100). C, Representative histologic sections of CIN2/3 epithelium with very sparse CD8 infiltrates (left panel) and MAdCAM-1 expression in a serial section, same field (right panel) (original magnification ×100). D, Quantification of MAdCAM-1 immunostaining in normal cervical epithelium (n = 7), normal cervical stroma (n = 8), CIN2/3 epithelium (n = 9), and CIN2/3 stroma (n = 9). E, Within-specimen correlation of the intensity of MAdCAM-1 and CD8 expression in normal and CIN2/3 mucosa. Each symbol represents the density of MAdCAM-1 (x coordinate) and CD8 (y coordinate) in the same field. The overall correlation is 0.738; correlation in CIN2/3 epithelium is 0.8833. p = 0.0031.
Similar articles
- Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions.
Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, Desmarais C, Boyer JD, Tycko B, Robins HS, Clark RA, Trimble CL. Maldonado L, et al. Sci Transl Med. 2014 Jan 29;6(221):221ra13. doi: 10.1126/scitranslmed.3007323. Sci Transl Med. 2014. PMID: 24477000 Free PMC article. Clinical Trial. - Naturally occurring systemic immune responses to HPV antigens do not predict regression of CIN2/3.
Trimble CL, Peng S, Thoburn C, Kos F, Wu TC. Trimble CL, et al. Cancer Immunol Immunother. 2010 May;59(5):799-803. doi: 10.1007/s00262-009-0806-4. Epub 2009 Dec 13. Cancer Immunol Immunother. 2010. PMID: 20012604 Free PMC article. - Vaccination against Oncoproteins of HPV16 for Noninvasive Vulvar/Vaginal Lesions: Lesion Clearance Is Related to the Strength of the T-Cell Response.
van Poelgeest MI, Welters MJ, Vermeij R, Stynenbosch LF, Loof NM, Berends-van der Meer DM, Löwik MJ, Hamming IL, van Esch EM, Hellebrekers BW, van Beurden M, Schreuder HW, Kagie MJ, Trimbos JB, Fathers LM, Daemen T, Hollema H, Valentijn AR, Oostendorp J, Oude Elberink JH, Fleuren GJ, Bosse T, Kenter GG, Stijnen T, Nijman HW, Melief CJ, van der Burg SH. van Poelgeest MI, et al. Clin Cancer Res. 2016 May 15;22(10):2342-50. doi: 10.1158/1078-0432.CCR-15-2594. Epub 2016 Jan 26. Clin Cancer Res. 2016. PMID: 26813357 Clinical Trial. - Correlation of E6 and E7 levels in high-risk HPV16 type cervical lesions with CCL20 and Langerhans cells.
Jiang B, Xue M. Jiang B, et al. Genet Mol Res. 2015 Sep 8;14(3):10473-81. doi: 10.4238/2015.September.8.8. Genet Mol Res. 2015. PMID: 26400278 - Principles of epithelial homeostasis control during persistent human papillomavirus infection and its deregulation at the cervical transformation zone.
Doorbar J, Zheng K, Aiyenuro A, Yin W, Walker CM, Chen Y, Egawa N, Griffin HM. Doorbar J, et al. Curr Opin Virol. 2021 Dec;51:96-105. doi: 10.1016/j.coviro.2021.09.014. Epub 2021 Oct 8. Curr Opin Virol. 2021. PMID: 34628359 Review.
Cited by
- T Cell Receptor Repertoires Acquired via Routine Pap Testing May Help Refine Cervical Cancer and Precancer Risk Estimates.
Christley S, Ostmeyer J, Quirk L, Zhang W, Sirak B, Giuliano AR, Zhang S, Monson N, Tiro J, Lucas E, Cowell LG. Christley S, et al. Front Immunol. 2021 Apr 2;12:624230. doi: 10.3389/fimmu.2021.624230. eCollection 2021. Front Immunol. 2021. PMID: 33868241 Free PMC article. - Moving forward with human papillomavirus immunotherapies.
Çuburu N, Schiller JT. Çuburu N, et al. Hum Vaccin Immunother. 2016 Nov;12(11):2875-2880. doi: 10.1080/21645515.2016.1199302. Epub 2016 Jul 7. Hum Vaccin Immunother. 2016. PMID: 27388123 Free PMC article. - Optimizing viable leukocyte sampling from the female genital tract for clinical trials: an international multi-site study.
McKinnon LR, Hughes SM, De Rosa SC, Martinson JA, Plants J, Brady KE, Gumbi PP, Adams DJ, Vojtech L, Galloway CG, Fialkow M, Lentz G, Gao D, Shu Z, Nyanga B, Izulla P, Kimani J, Kimwaki S, Bere A, Moodie Z, Landay AL, Passmore JA, Kaul R, Novak RM, McElrath MJ, Hladik F. McKinnon LR, et al. PLoS One. 2014 Jan 15;9(1):e85675. doi: 10.1371/journal.pone.0085675. eCollection 2014. PLoS One. 2014. PMID: 24454917 Free PMC article. - Microparticles produced by human papillomavirus type 16 E7-expressing cells impair antigen presenting cell function and the cytotoxic T cell response.
Zhang J, Burn C, Young K, Wilson M, Ly K, Budhwani M, Tschirley A, Braithwaite A, Baird M, Hibma M. Zhang J, et al. Sci Rep. 2018 Feb 5;8(1):2373. doi: 10.1038/s41598-018-20779-2. Sci Rep. 2018. PMID: 29402982 Free PMC article. - Memory CD8+ T cell responses to cancer.
Han J, Khatwani N, Searles TG, Turk MJ, Angeles CV. Han J, et al. Semin Immunol. 2020 Jun;49:101435. doi: 10.1016/j.smim.2020.101435. Epub 2020 Nov 30. Semin Immunol. 2020. PMID: 33272898 Free PMC article. Review.
References
- Frazer IH, Lowy DR, Schiller JT. Prevention of cancer through immunization: Prospects and challenges for the 21st century. Eur J Immunol. 2007;37(Suppl 1):S148–S155. - PubMed
- Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. - PubMed
- Fleurence RL, Dixon JM, Milanova TF, Beusterien KM. Review of the economic and quality-of-life burden of cervical human papillomavirus disease. Am J Obstet Gynecol. 2007;196:206–212. - PubMed
- Monk BJ, Herzog TJ. The evolution of cost-effective screening and prevention of cervical carcinoma: implications of the 2006 consensus guidelines and human papillomavirus vaccination. Am J Obstet Gynecol. 2007;197:337–339. - PubMed
- Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus—related disease. Am J Obstet Gynecol. 2004;191:114–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA142691-01A1/CA/NCI NIH HHS/United States
- R01 CA142691/CA/NCI NIH HHS/United States
- K08 AI060890/AI/NIAID NIH HHS/United States
- R01 AR056720/AR/NIAMS NIH HHS/United States
- K23 CA085437/CA/NCI NIH HHS/United States
- 1K23CA85437/CA/NCI NIH HHS/United States
- K23 CA085437-05/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials