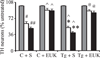Synergistic effects of environmental risk factors and gene mutations in Parkinson's disease accelerate age-related neurodegeneration - PubMed (original) (raw)
Comparative Study
Synergistic effects of environmental risk factors and gene mutations in Parkinson's disease accelerate age-related neurodegeneration
Jun Peng et al. J Neurochem. 2010 Dec.
Abstract
As Parkinson's disease appears to be a multifactoral disorder, the use of animal models to investigate combined effects of genetic and environmental risk factors are of great importance especially in the context of aging which is the single major risk factor for the disorder. Here, we assessed the combined effects of neonatal iron feeding and environmental paraquat exposure on age-related nigrostriatal degeneration in transgenic mice expressing the A53T familial mutant form of human α-synuclein within these neurons. We report here that A53T α-synuclein mice exhibit greater susceptibility to paraquat. Increased oral intake of iron in the neonatal period leads to a progressive age-related enhancement of dopaminergic neurodegeneration associated with paraquat neurotoxicity. Furthermore, neurodegeneration associated with these combined genetic and environmental risk factors could be attenuated by systemic treatment with the bioavailable antioxidant compound EUK-189. These data suggest that environmental factors previously identified as contributors to neurodegeneration associated with sporadic Parkinson's disease may also be candidates for observed variations in symptoms and disease progression in monogenic forms and that this may mechanistically involve increased levels of oxidatively-induced post-translational nitration of α-synuclein.
© 2010 The Authors. Journal of Neurochemistry © 2010 International Society for Neurochemistry.
Figures
Fig. 1
Schematic of experimental paradigms. (a) Saline or carbonyl iron was administered to mice from postnatal days 10 to 17 via oral gavage. Mice were aged to 2, 12, and 23 months of age and were intraperitoneally injected with saline or paraquat twice per week for 3 weeks. Animals were killed at day 7 after final administration. (b) Pumps were implanted 3 days prior to paraquat treatment.
Fig. 2
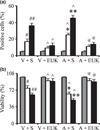
Mutant α-synuclein over-expression enhances the affects of combined iron-paraquat-induced apoptosis in a dopaminergic cell line. α-Synuclein transfected N27 cells were treated with 350 µM paraquat and 80 µM FeCl2 for 24 h. Cells were treated with EUK-189 1 h prior to the addition of FeCl2 and paraquat. (a) TUNEL-positive cells were measured at 24 h. (b) Cell viability was measured via the MTT assay and expressed as a percentage of viability in control at 24 h. Gray bars, saline; white bars, paraquat alone; and black bars, paraquat plus FeCl2. Mean ± SEM, n = 5. #p < 0.01, significantly different from vector plus saline plus saline; ##p < 0.05, significantly different from vector plus saline plus paraquat; ^p < 0.05, significantly different from matched vector plus saline; *p < 0.05, significantly different from A53T plus saline plus saline; **p < 0.05, significantly different from A53T plus saline plus paraquat; and @p < 0.01, significantly different from matched A53T plus saline. V + S, vector plus saline; V + EUK, vector plus EUK-189; A + S, A53T plus saline; A + E, A53T plus EUK-189.
Fig. 3
Mutant α-synuclein over-expression enhances combined iron-paraquat-induced dopaminergic neuron death in primary mesencephalic cultures. Cultures from control or A53T over-expression mice were treated with 30 µM paraquat without or with 8 µM FeCl2. Cultures were treated with EUK-189 1 h prior to the addition of paraquat and FeCl2. TH-positive neuron counts in mesencephalic cultures 24 h after paraquat without or with FeCl2 treatment. Gray bars, saline; white bars, paraquat alone; and black bars, paraquat plus FeCl2. Mean ± SEM, n = 4. #p < 0.01, significantly different from control plus saline plus saline; ## p < 0.05, significantly different from control plus saline plus paraquat; ^p < 0.05, significantly different from matched control plus saline; *p < 0.01, significantly different from A53T plus saline plus saline; **p < 0.05, significantly different from A53T plus saline plus paraquat; and @p < 0.01, significantly different from matched A53T plus saline. C + S, control plus saline; C + EUK, control plus EUK-189; Tg + S, transgenic A53T plus saline; Tg + E, transgenic A53T plus EUK-189.
Fig. 4
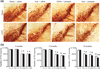
Effects of combined environmental and genetic factors on dopaminergic neurodegeneration with increasing age. (a) Photomicrographs of SNpc TH-immunostained sections from 23-month-old control versus A53T transgenic mice treated with either paraquat or iron alone or in combination. Original magnification, ×10. (b) Quantitative stereological analysis of the number of TH-stained profiles from 2-month-old, 12-month-old, and 23-month-old mice. Mean ± SEM, the number of animal per group is 4 or 5. White bars, control; black bars, A53T over-expression. #p < 0.01, significantly different from control plus saline plus saline group;*p < 0.01, significantly different from A53T plus saline plus saline group; ##p < 0.05, significantly different from control plus saline plus paraquat group;**p < 0.05, significantly different from A53T plus saline plus paraquat group; and ^p < 0.05, significantly different from matched control group. S + S, saline plus saline; Fe + S, iron plus saline; S + PQ, saline plus paraquat; Fe + PQ, iron plus paraquat.
Fig. 5
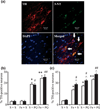
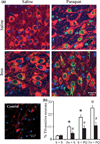
3-Nitrotyrosine immunopositive SNpc cell counts. (a) An example of localization (arrows) of 3-nitrotyrosine-immunopositive staining (green) within dopaminergic neurons (red). 4′,6-Diamidino-2-phenylindole (blue) was used to counter-stain nuclei. Scale bar, 20 µm. Quantitative analysis of double labeling for TH with 3-nitrotyrosine in the SNpc of 2-month-old (b) and 23-month-old (c) mice. White bars, control; black bars, A53T over-expression. Mean ± SEM, the number of animal per group is 4 or 5. *p < 0.01, significantly different from control plus saline plus saline group; **p < 0.05, significantly different from control plus saline plus paraquat group; #p < 0.01, significantly different from A53T plus saline plus saline group; ##p < 0.05, significantly different from A53T plus saline plus paraquat group; and ^p < 0.05, significantly different from matched control group. S + S, saline plus saline; Fe + S, iron plus saline; S + PQ, saline plus paraquat; Fe + PQ, iron plus paraquat.
Fig. 6
Administration of EUK-189 attenuates the exacerbation of combined neonatal iron-paraquat-induced dopaminergic neuronal death at 12 months of age in the A53T expressing mice. (a) Photomicrographs of SNpc TH-immunostained sections. Original magnification, ×10. Quantitative stereological analysis of the number of TH-stained profiles from control (b) and A53T over-expression (c) mice. Quantitative analysis of double labeling for TH with 3-nitrotyrosine in the SNpc of control (d) and A53T over-expression (e) mice. White bars, vehicle and black bars, EUK-189-treated. Mean ± SEM, the number of animal per group is 3 or 4. *p < 0.01, significantly different from saline plus saline plus vehicle group; @p < 0.05, significantly different from saline plus paraquat plus vehicle group; #p < 0.01, significantly different from matched vehicle group. S + S, saline plus saline; Fe + S, iron plus saline; S + PQ, saline plus paraquat; Fe + PQ, iron plus paraquat.
Fig. 7
Administration of EUK-189 prevents α-synuclein nitration in the SNpc. (a) Nitrated α-synuclein-immunopositive staining (green) within dopaminergic neurons (red). 4′,6-Diamidino-2-phenylindole (blue) was used to counter-stain nuclei. Scale bar, 20 µm. (b) Quantitative analysis of double labeling for TH with nitrated α-synuclein in the SNpc of 12-month-old A53T expressing transgenics. White bars, vehicle and black bars, EUK-189-treated. Mean ± SEM, the number of animal per group is 3 or 4. *p < 0.001, significantly different from saline plus saline plus vehicle group; @p < 0.05, significantly different from saline plus paraquat plus vehicle group; #p < 0.001, significantly different from matched vehicle group. S + S, saline plus saline; Fe + S, iron plus saline; S + PQ, saline plus paraquat; Fe + PQ, iron plus paraquat.
Similar articles
- Iron and paraquat as synergistic environmental risk factors in sporadic Parkinson's disease accelerate age-related neurodegeneration.
Peng J, Peng L, Stevenson FF, Doctrow SR, Andersen JK. Peng J, et al. J Neurosci. 2007 Jun 27;27(26):6914-22. doi: 10.1523/JNEUROSCI.1569-07.2007. J Neurosci. 2007. PMID: 17596439 Free PMC article. - DNA damage preceding dopamine neuron degeneration in A53T human α-synuclein transgenic mice.
Wang D, Yu T, Liu Y, Yan J, Guo Y, Jing Y, Yang X, Song Y, Tian Y. Wang D, et al. Biochem Biophys Res Commun. 2016 Dec 2;481(1-2):104-110. doi: 10.1016/j.bbrc.2016.11.008. Epub 2016 Nov 3. Biochem Biophys Res Commun. 2016. PMID: 27818201 - Chronic mild stress accelerates the progression of Parkinson's disease in A53T α-synuclein transgenic mice.
Wu Q, Yang X, Zhang Y, Zhang L, Feng L. Wu Q, et al. Exp Neurol. 2016 Nov;285(Pt A):61-71. doi: 10.1016/j.expneurol.2016.09.004. Epub 2016 Sep 13. Exp Neurol. 2016. PMID: 27637804 - Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder?
Olanow CW, Brundin P. Olanow CW, et al. Mov Disord. 2013 Jan;28(1):31-40. doi: 10.1002/mds.25373. Mov Disord. 2013. PMID: 23390095 Review.
Cited by
- Co-administration of Nanowired DL-3-n-Butylphthalide (DL-NBP) Together with Mesenchymal Stem Cells, Monoclonal Antibodies to Alpha Synuclein and TDP-43 (TAR DNA-Binding Protein 43) Enhance Superior Neuroprotection in Parkinson's Disease Following Concussive Head Injury.
Feng L, Sharma A, Wang Z, Muresanu DF, Tian ZR, Lafuente JV, Buzoianu AD, Nozari A, Wiklund L, Sharma HS. Feng L, et al. Adv Neurobiol. 2023;32:97-138. doi: 10.1007/978-3-031-32997-5_3. Adv Neurobiol. 2023. PMID: 37480460 Review. - Patient-specific pluripotent stem cell-based Parkinson's disease models showing endogenous alpha-synuclein aggregation.
Oh Y. Oh Y. BMB Rep. 2019 Jun;52(6):349-359. doi: 10.5483/BMBRep.2019.52.6.142. BMB Rep. 2019. PMID: 31186086 Free PMC article. Review. - PARK2 patient neuroprogenitors show increased mitochondrial sensitivity to copper.
Aboud AA, Tidball AM, Kumar KK, Neely MD, Han B, Ess KC, Hong CC, Erikson KM, Hedera P, Bowman AB. Aboud AA, et al. Neurobiol Dis. 2015 Jan;73:204-12. doi: 10.1016/j.nbd.2014.10.002. Epub 2014 Oct 12. Neurobiol Dis. 2015. PMID: 25315681 Free PMC article. - Allopregnanolone reinstates tyrosine hydroxylase immunoreactive neurons and motor performance in an MPTP-lesioned mouse model of Parkinson's disease.
Adeosun SO, Hou X, Jiao Y, Zheng B, Henry S, Hill R, He Z, Pani A, Kyle P, Ou X, Mosley T, Farley JM, Stockmeier C, Paul I, Bigler S, Brinton RD, Smeyne R, Wang JM. Adeosun SO, et al. PLoS One. 2012;7(11):e50040. doi: 10.1371/journal.pone.0050040. Epub 2012 Nov 29. PLoS One. 2012. PMID: 23209637 Free PMC article. - Oxidative stress in genetic mouse models of Parkinson's disease.
Varçin M, Bentea E, Michotte Y, Sarre S. Varçin M, et al. Oxid Med Cell Longev. 2012;2012:624925. doi: 10.1155/2012/624925. Epub 2012 Jul 8. Oxid Med Cell Longev. 2012. PMID: 22829959 Free PMC article. Review.
References
- Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J. Neurochem. 1997;69:1326–1329. - PubMed
- Baker K, Marcus CB, Huffman K, et al. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J. Pharmacol. Exp. Ther. 1998;284:215–221. - PubMed
- Baudry M, Etienne S, Bruce A, Palucki M, Jacobsen E, Malfroy B. Salen-manganese complexes are superoxide dismutasemimics. Biochem. Biophys. Res. Commun. 1993;192:964–968. - PubMed
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. - PubMed
- Burkitt MJ, Kadiiska MB, Hanna PM, Jordan SJ, Mason RP. Electron spin resonance spin-trapping investigation into the effects of paraquat and desferrioxamine on hydroxyl radical generation during acute iron poisoning. Mol. Pharmacol. 1993;43:257–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials