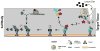Subcellular communication through RNA transport and localized protein synthesis - PubMed (original) (raw)
Review
Subcellular communication through RNA transport and localized protein synthesis
Christopher J Donnelly et al. Traffic. 2010 Dec.
Abstract
Interest in the mechanisms of subcellular localization of mRNAs and the effects of localized translation has increased over the last decade. Polarized eukaryotic cells transport mRNA-protein complexes to subcellular sites, where translation of the mRNAs can be regulated by physiological stimuli. The long distances separating distal neuronal processes from their cell body have made neurons a useful model system for dissecting mechanisms of mRNA trafficking. Both the dendritic and axonal processes of neurons have been shown to have protein synthetic capacity and the diversity of mRNAs discovered in these processes continues to increase. Localized translation of mRNAs requires a co-ordinated effort by the cell body to target both mRNAs and necessary translational machinery into distal sites, as well as temporal control of individual mRNA translation. In addition to altering protein composition locally at the site of translation, some of the proteins generated in injured nerves retrogradely signal to the cell body, providing both temporal and spatial information on events occurring at distant subcellular sites.
© 2010 John Wiley & Sons A/S.
Figures
Figure 1. Spatial signaling of nerve injury through localized mRNA translation
The schematic illustrates a segment of distal axon from proximal (left) to distal (right]. Injury to the axon causes an increase in axoplasmic [Ca2+] from influx and release of intracellular stores [1]. This triggers translation of axonal mRNAs including Importin β1, RanBP1, and vimentin [3]. The newly synthesized RanBP1 causes dissociation of the Importin α complex that includes Ran-GAP [2,4]. Importin α is then able to heterodimerize with newly synthsized Importin β1 [5]. Ca2+-triggered protease activity cleaves the newly synthesized Vimentin protein [6], generating a scaffold to link injury-activated Erks to the Importin α/β1 complex for retrograde transport to the cell body through the dynein motor proteins [7].
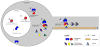
Figure 2. Sequential assembly of transport RNPs through mRNA-protein interactions
β-actin mRNA targeting is used as an example here to illustrate sequential assembly of RNA transport granule or RNPs. The KHSRP protein, ZBP2, initially binds to nascent β-actin transcript in the nucleus [2]. This facilitates binding of ZBP1 in the nucleus [3], with the ZBP1-β-actin complex eventually localizing into distal cytoplasmic sites through interaction with microtubule motor proteins in the cytoplasm [6]. This interaction is presumably as part of a large RNP complex with additional protein linking ZBP1-β-actin to the plus-ended kinesin motor protein, but also sequester the mRNA(s) from translational machinery [4 & 5]. By definition, ZBP1 can shuttle between the cytoplasm and nucleus [1]; however it is not clear whether ZBP1 is recycled from distal cytoplasmic processes back to the nucleus.
Similar articles
- Regulation of protein levels in subcellular domains through mRNA transport and localized translation.
Willis DE, Twiss JL. Willis DE, et al. Mol Cell Proteomics. 2010 May;9(5):952-62. doi: 10.1074/mcp.R900005-MCP200. Epub 2010 Feb 18. Mol Cell Proteomics. 2010. PMID: 20167945 Free PMC article. - RNA transport and localized protein synthesis in neurological disorders and neural repair.
Wang W, van Niekerk E, Willis DE, Twiss JL. Wang W, et al. Dev Neurobiol. 2007 Aug;67(9):1166-82. doi: 10.1002/dneu.20511. Dev Neurobiol. 2007. PMID: 17514714 Review. - Dynamics of axonal mRNA transport and implications for peripheral nerve regeneration.
Yoo S, van Niekerk EA, Merianda TT, Twiss JL. Yoo S, et al. Exp Neurol. 2010 May;223(1):19-27. doi: 10.1016/j.expneurol.2009.08.011. Epub 2009 Aug 20. Exp Neurol. 2010. PMID: 19699200 Free PMC article. Review. - Cataloguing and Selection of mRNAs Localized to Dendrites in Neurons and Regulated by RNA-Binding Proteins in RNA Granules.
Ohashi R, Shiina N. Ohashi R, et al. Biomolecules. 2020 Jan 22;10(2):167. doi: 10.3390/biom10020167. Biomolecules. 2020. PMID: 31978946 Free PMC article. Review. - Profiling axonal mRNA transport.
Willis DE, Twiss JL. Willis DE, et al. Methods Mol Biol. 2011;714:335-52. doi: 10.1007/978-1-61779-005-8_21. Methods Mol Biol. 2011. PMID: 21431751 Free PMC article.
Cited by
- MicroRNAs shape the neuronal landscape.
McNeill E, Van Vactor D. McNeill E, et al. Neuron. 2012 Aug 9;75(3):363-79. doi: 10.1016/j.neuron.2012.07.005. Neuron. 2012. PMID: 22884321 Free PMC article. Review. - GM-lncLoc: LncRNAs subcellular localization prediction based on graph neural network with meta-learning.
Cai J, Wang T, Deng X, Tang L, Liu L. Cai J, et al. BMC Genomics. 2023 Jan 28;24(1):52. doi: 10.1186/s12864-022-09034-1. BMC Genomics. 2023. PMID: 36709266 Free PMC article. - Increases in Retrograde Injury Signaling Complex-Related Transcripts in Central Axons following Injury.
Pathak GK, Ornstein H, Aranda-Espinoza H, Karlsson AJ, Shah SB. Pathak GK, et al. Neural Plast. 2016;2016:3572506. doi: 10.1155/2016/3572506. Epub 2016 Oct 25. Neural Plast. 2016. PMID: 27847648 Free PMC article. - Local gene expression changes after UV-irradiation of human skin.
Weinkauf B, Rukwied R, Quiding H, Dahllund L, Johansson P, Schmelz M. Weinkauf B, et al. PLoS One. 2012;7(6):e39411. doi: 10.1371/journal.pone.0039411. Epub 2012 Jun 22. PLoS One. 2012. PMID: 22761785 Free PMC article. - Lysophosphatidic acid differentially regulates axonal mRNA translation through 5'UTR elements.
Vuppalanchi D, Merianda TT, Donnelly C, Pacheco A, Williams G, Yoo S, Ratan RR, Willis DE, Twiss JL. Vuppalanchi D, et al. Mol Cell Neurosci. 2012 Jun;50(2):136-46. doi: 10.1016/j.mcn.2012.04.001. Epub 2012 Apr 10. Mol Cell Neurosci. 2012. PMID: 22522146 Free PMC article.
References
- Lecuyer E, Yoshida H, Parthasarathy N, Alm C, Babak T, Cerovina T, Hughes TR, Tomancak P, Krause HM. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell. 2007;131(1):174–187. - PubMed
- Paquin N, Chartrand P. Local regulation of mRNA translation: new insights from the bud. Trends Cell Biol. 2008;18(3):105–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS049041/NS/NINDS NIH HHS/United States
- R01 NS049041-04/NS/NINDS NIH HHS/United States
- R01-NS041596/NS/NINDS NIH HHS/United States
- R01 NS041596-09/NS/NINDS NIH HHS/United States
- R01-NS049041/NS/NINDS NIH HHS/United States
- R01 NS041596/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources