Diverse mechanisms of Wnt activation and effects of pathway inhibition on proliferation of human gastric carcinoma cells - PubMed (original) (raw)
Diverse mechanisms of Wnt activation and effects of pathway inhibition on proliferation of human gastric carcinoma cells
S Asciutti et al. Oncogene. 2011.
Abstract
Human gastric carcinomas are among the most treatment-refractory epithelial malignancies. Increased understanding of the underlying molecular aberrations in such tumors could provide insights leading to improved therapeutic approaches. In this study, we characterized diverse genetic aberrations leading to constitutive Wnt signaling activation in a series of human gastric carcinoma cell lines. Downregulation of TCF signaling by stable transduction of dominant negative TCF4 (DNTCF4) resulted in inhibition of proliferation in Wnt-activated AGS tumor cells. c-Myc downregulation and the associated upregulation of its repression target, p21 observed in these tumor cells, as well as the profound growth inhibition induced by c-Myc small hairpin RNA (shRNA) implied their c-Myc addiction. In striking contrast, Wnt-activated MKN-28 and MKN-74 tumor cells appeared refractory to DNTCF4 inhibition of proliferation despite comparably decreased c-Myc expression levels. The resistance of these same tumor cells to growth inhibition by c-Myc shRNA established that their refractoriness to DNTCF was because of their independence from c-Myc for proliferation. There was no correlation between this resistance phenotype and the presence or absence of constitutive mitogen-activated protein kinase (MAPK) and/or AKT pathway activation, commonly observed in gastrointestinal tumors. However, in both DNTCF-sensitive and -resistant tumor cells with MAPK and/or AKT pathway activation, the ability of small molecule antagonists directed against either pathway to inhibit tumor cell growth was enhanced by Wnt pathway inhibition. These findings support the concept that although certain Wnt-activated tumors may escape c-Myc dependence for proliferation, disruption of other oncogenic pathways can unmask cooperative antiproliferative effects for Wnt pathway downregulation.
Conflict of interest statement
Conflict of interest
No conflicts of interest to disclose.
Figures
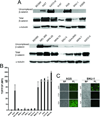
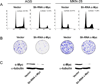
Figure 1. Wnt signaling upregulation in human gastric tumor cell lines
(A) 1 mg of total cell lysates was subjected to GST-E-cadherin pull down assay (Bafico et al., 1998). Uncomplexed β-catenin and total cell lysates (0.1 mg) were immunobloted using an anti-β-catenin mAb. (B) TCF-GFP reporter activity in human gastric cell lines. Cells were infected with TOP or FOP GFP lentiviruses as described in materials and methods. Columns represent TOP/FOP Mean Fluorescence Intensity (MFI). Infections were performed in triplicates. (C) Phase contrast and fluorescence images of AGS and SNU-1 cells infected with TOP or FOP TCF-GFP reporter lentiviruses or with GFP expressing lentivirus. BF - Bright Field, FL - Fluorescence.
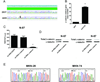
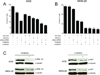
Figure 2. Mechanisms involved in TCF activity in gastric tumor cell lines
(A) Sequencing of CTNNB1 (β-catenin) gene in 293T and AGS cells. AGS cell line shows a missense mutation in codon 34. (B) Analysis of CTNNB1 gene copy number using real time PCR (RT-PCR) analysis. Results are depicted as gene copies relative to 293T cells. Error bars indicate S.D. (*)=p<0.05. (C) Effects of shRNA knockdown of either β or γ-catenin on TCF-Luciferase (Luc) activity in N-87 cells. ShRNA targeting keratinocyte growth factor receptor (KGFR) was used as control. Data represent the mean ± S.D. of three independent experiments. (D) Efficiency of lentiviral mediated shRNA knockdown of total β or γ-catenin in N-87 cells. ShRNA targeting KGFR was used as control. (E) Sequencing of exon 15 of APC gene in MKN-28 and MKN-74 cells. Both MKN-28 and MKN-74 cell lines show “C” to “T” base change leading conversion of R1450 to a stop codon.
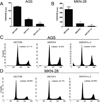
Figure 3. Effects of Wnt pathway inhibition on TCF reporter activity and cell cycle profile in AGS and MKN-28 cell lines
Analysis of TOP/FOP TCF-Luciferase (Luc) activity in AGS (A) and MKN-28 (B) cells following one or two cycles of transduction using lentiviruses expressing VECTOR or DNTCF4. Data represent the mean ± S.D. of three independent experiments performed in duplicates. (*)=p<0.05, VECTOR vs. DNTCF4 (one cycle of transduction). (**)=p<0.05, VECTOR vs DNTCF4, (two cycles of transduction). Representative FACS analysis cell cycle profiles of propidium iodide (PI) stained AGS (C) and MKN-28 (D) cells following one or two cycles of transduction with either VECTOR or DNTCF4 expressing lentiviruses.
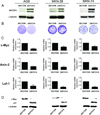
Figure 4. Effects of TCF signaling inhibition on cell proliferation of gastric cell lines
(A) Immunoblot analysis of DNTCF4 expression in AGS, MKN-28, and MKN-74 cells infected with either VECTOR or DNTCF4 expressing lentiviruses. DNTCF4 expression was detected using an antibody to TCF-4. (B) Effects of DNTCF4 on cell growth of AGS, MKN-28, and MKN-74 cells. For long term growth, cells were seeded in 60 mm dishes and cultures visualized by crystal violet staining after 14 days. (*)=p<0.05. (C) Effects of DNTCF4 on expression of TCF target genes. RT-PCR analysis of c-Myc, Axin-2, and Lef-1 mRNA levels in AGS, MKN-28, and MKN-74 cells transduced with VECTOR or DNTCF4 expressing lentiviruses. Error bars indicate S.D. (*)=p<0.05. (D) Effects of DNTCF4 on expression of c-Myc, and p21 protein levels in AGS, MKN-28, and MKN-74 cells transduced with VECTOR or DNTCF4 expressing lentiviruses.
Figure 5. Effects of c-Myc shRNA signaling inhibition on proliferation of AGS and MKN-28 gastrointestinal tumor lines
(A) Representative FACS analysis cell cycle profiles of propidium iodide (PI) stained AGS and MKN-28 cells following infection with lentiviral vectors expressing control or c-Myc-shRNA. (B) Effects of c-Myc-shRNA on cell growth of AGS, and MKN-28 cells. For colony formation, 2×103 cells were seeded in 6-well plates, marker selected with puromycin, and cultures visualized by crystal violet staining after 14 days. (*)=p<0.05. (C) Efficiency of lentiviral mediated shRNA knockdown of c-Myc in AGS and MKN-28 cells.
Figure 6. Effects of combined downregulation of Wnt together with MAPK or PI3K/AKT pathways on gastric tumor cell growth
(A, B) AGS and MKN-28 cells transduced with VECTOR, DNTCF4, or c-Myc shRNA were treated with either LY294002 (40 µM), PD98059 (40 µM), or DMSO for 36 h. Cells were analyzed for cell cycle profile by FACS analysis. (*)=p<0.05, VECTOR vs. DNTCF4 or c-Myc shRNA. (**)=p<0.05, DNTCF4 alone vs. DNTCF4 + LY294002 or PD98059. (***)=p<0.05, c-Myc shRNA alone vs. c-Myc shRNA + LY294002 or PD98059. Data represent the mean ± S.D. from three independent experiments. (C) Immunoblot analysis of pERK and pAKT protein levels in AGS, and MKN-28 cells treated with PD98059 or LY294002.
Similar articles
- Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo.
Ramachandran I, Thavathiru E, Ramalingam S, Natarajan G, Mills WK, Benbrook DM, Zuna R, Lightfoot S, Reis A, Anant S, Queimado L. Ramachandran I, et al. Oncogene. 2012 May 31;31(22):2725-37. doi: 10.1038/onc.2011.455. Epub 2011 Oct 17. Oncogene. 2012. PMID: 22002305 - Capsosiphon fulvescens glycoprotein inhibits AGS gastric cancer cell proliferation by downregulating Wnt-1 signaling.
Kim YM, Kim IH, Nam TJ. Kim YM, et al. Int J Oncol. 2013 Nov;43(5):1395-401. doi: 10.3892/ijo.2013.2079. Epub 2013 Aug 23. Int J Oncol. 2013. PMID: 23982808 Free PMC article. - HNF4α is a therapeutic target that links AMPK to WNT signalling in early-stage gastric cancer.
Chang HR, Nam S, Kook MC, Kim KT, Liu X, Yao H, Jung HR, Lemos R Jr, Seo HH, Park HS, Gim Y, Hong D, Huh I, Kim YW, Tan D, Liu CG, Powis G, Park T, Liang H, Kim YH. Chang HR, et al. Gut. 2016 Jan;65(1):19-32. doi: 10.1136/gutjnl-2014-307918. Epub 2014 Nov 19. Gut. 2016. PMID: 25410163 Free PMC article. - Wnt/β-catenin, an oncogenic pathway targeted by H. pylori in gastric carcinogenesis.
Song X, Xin N, Wang W, Zhao C. Song X, et al. Oncotarget. 2015 Nov 3;6(34):35579-88. doi: 10.18632/oncotarget.5758. Oncotarget. 2015. PMID: 26417932 Free PMC article. Review. - Turning the tables: Myc activates Wnt in breast cancer.
Cowling VH, Cole MD. Cowling VH, et al. Cell Cycle. 2007 Nov 1;6(21):2625-7. doi: 10.4161/cc.6.21.4880. Epub 2007 Aug 13. Cell Cycle. 2007. PMID: 17726380 Review.
Cited by
- Landscape of Druggable Molecular Pathways Downstream of Genomic CDH1/Cadherin-1 Alterations in Gastric Cancer.
Malpeli G, Barbi S, Innamorati G, Alloggio M, Filippini F, Decimo I, Castelli C, Perris R, Bencivenga M. Malpeli G, et al. J Pers Med. 2022 Dec 3;12(12):2006. doi: 10.3390/jpm12122006. J Pers Med. 2022. PMID: 36556227 Free PMC article. - Computational Drug Repositioning and Experimental Validation of Ivermectin in Treatment of Gastric Cancer.
Rabben HL, Andersen GT, Ianevski A, Olsen MK, Kainov D, Grønbech JE, Wang TC, Chen D, Zhao CM. Rabben HL, et al. Front Pharmacol. 2021 Mar 31;12:625991. doi: 10.3389/fphar.2021.625991. eCollection 2021. Front Pharmacol. 2021. PMID: 33867984 Free PMC article. - Discovery of Drug Synergies in Gastric Cancer Cells Predicted by Logical Modeling.
Flobak Å, Baudot A, Remy E, Thommesen L, Thieffry D, Kuiper M, Lægreid A. Flobak Å, et al. PLoS Comput Biol. 2015 Aug 28;11(8):e1004426. doi: 10.1371/journal.pcbi.1004426. eCollection 2015 Aug. PLoS Comput Biol. 2015. PMID: 26317215 Free PMC article. - Quantitative analysis of the TNF-α-induced phosphoproteome reveals AEG-1/MTDH/LYRIC as an IKKβ substrate.
Krishnan RK, Nolte H, Sun T, Kaur H, Sreenivasan K, Looso M, Offermanns S, Krüger M, Swiercz JM. Krishnan RK, et al. Nat Commun. 2015 Apr 7;6:6658. doi: 10.1038/ncomms7658. Nat Commun. 2015. PMID: 25849741 Free PMC article. - High-resolution chromatin immunoprecipitation (ChIP) sequencing reveals novel binding targets and prognostic role for SOX11 in mantle cell lymphoma.
Kuo PY, Leshchenko VV, Fazzari MJ, Perumal D, Gellen T, He T, Iqbal J, Baumgartner-Wennerholm S, Nygren L, Zhang F, Zhang W, Suh KS, Goy A, Yang DT, Chan WC, Kahl BS, Verma AK, Gascoyne RD, Kimby E, Sander B, Ye BH, Melnick AM, Parekh S. Kuo PY, et al. Oncogene. 2015 Mar 5;34(10):1231-40. doi: 10.1038/onc.2014.44. Epub 2014 Mar 31. Oncogene. 2015. PMID: 24681958 Free PMC article.
References
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 Is a Specific Inhibitor of the Activation of Mitogen-activated Protein Kinase Kinase in Vitro and in Vivo. Journal of Biological Chemistry. 1995;270:27489–27494. - PubMed
- Bafico A, Gazit A, Wu-Morgan SS, Yaniv A, Aaronson SA. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene. 1998;16:2819–2825. - PubMed
- Bas PLW, Friedel N, Nico JDB, Hugo WT, Winand NMD. Genetic alterations involving exon 3 of the beta-catenin gene do not play a role in adenocarcinomas of the esophagus. International Journal of Cancer. 2000;86:533–537. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical