Lesson from the stoichiometry determination of the cohesin complex: a short protease mediated elution increases the recovery from cross-linked antibody-conjugated beads - PubMed (original) (raw)
. 2011 Feb 4;10(2):780-9.
doi: 10.1021/pr100927x. Epub 2010 Nov 18.
Affiliations
- PMID: 21043528
- PMCID: PMC3033704
- DOI: 10.1021/pr100927x
Free PMC article
Lesson from the stoichiometry determination of the cohesin complex: a short protease mediated elution increases the recovery from cross-linked antibody-conjugated beads
Johann Holzmann et al. J Proteome Res. 2011.
Free PMC article
Abstract
Affinity purification of proteins using antibodies coupled to beads and subsequent mass spectrometric analysis has become a standard technique for the identification of protein complexes. With the recent transfer of the isotope dilution mass spectrometry principle (IDMS) to the field of proteomics, quantitative analyses-such as the stoichiometry determination of protein complexes-have become achievable. Traditionally proteins were eluted from antibody-conjugated beads using glycine at low pH or using diluted acids such as HCl, TFA, or FA, but elution was often found to be incomplete. Using the cohesin complex and the anaphase promoting complex/cyclosome (APC/C) as examples, we show that a short 15-60 min predigestion with a protease such as LysC (modified on-bead digest termed protease elution) increases the elution efficiency 2- to 3-fold compared to standard acid elution protocols. While longer incubation periods-as performed in standard on-bead digestion-led to partial proteolysis of the cross-linked antibodies, no or only insignificant cleavage was observed after 15-60 min protease mediated elution. Using the protease elution method, we successfully determined the stoichiometry of the cohesin complex by absolute quantification of the four core subunits using LC-SRM analysis and 19 reference peptides generated with the EtEP strategy. Protease elution was 3-fold more efficient compared to HCl elution, but measurements using both elution techniques are in agreement with a 1:1:1:1 stoichiometry. Furthermore, using isoform specific reference peptides, we determined the exact STAG1:STAG2 stoichiometry within the population of cohesin complexes. In summary, we show that the protease elution protocol increases the recovery from affinity beads and is compatible with quantitative measurements such as the stoichiometry determination of protein complexes.
Figures
Figure 1
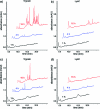
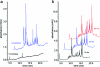
UV chromatograms of proteolyzed cross-linked antibodies analyzed using a monolithic column. αRAD21 (panels (a) and (b)) and αAPC3 (panels (c) and (d)) antibodies cross-linked to AffiPrep A beads were incubated with trypsin and LysC, respectively. At the indicated time points an aliquot, which corresponded to 2 pmol antibody (assuming complete cleavage) was separated using a monolithic column. Increasing absorption in the UV chromatograms indicate proteolytic cleavage of the antibodies. For better illustration, chromatograms are displayed with a 5% time and a 15% signal offset.
Figure 2
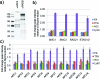
(a) SDS-PAGE of isolated APC/C and cohesin from soluble extract of HeLa cells. (b) Labelfree quantification of cohesin subunits eluted using different protocols by XIC relative to glycine elution. (c) Labelfree quantification of APC/C subunits eluted using different protocols by XIC relative to glycine elution. Data are mean ± SD of duplicate measurements. See also Figures S3 and S4 for peptide scattering within subunits in the technical duplicates (Supporting Information).
Figure 3
UV chromatograms of proteolyzed cross-linked and noncross-linked antibodies analyzed using a monolithic column. Cohesin was purified using αRAD21 antibody beads. (a) Cross-linked and noncross-linked αRAD21 antibody beads were incubated with LysC for 15 min and an aliquot, which corresponded to 2 pmol antibodies (assuming complete cleavage) was separated using a monolithic column. (b) 50-fold the amount shown in (a) (of cross-linked αRAD21 antibody beads) was separated using a monolithic column, after 15, 30, and 60 min of incubation with LysC. For better illustration chromatograms are displayed with a 5% time and a 15% signal offset.
Figure 4
Absolute quantification of isolated cohesin subunits by LC-SRM analysis on a 5500 QTRAP. (a) Comparing 15, 30, and 60 min LysC protease elution. Data are mean ± SD of four measured peptides in case of SMC1, SMC3 and RAD21 and of three measured peptides in case of STAG1 and STAG2, respectively. Measurements of cohesin peptides were performed in duplicate. (b) Calculation of complex stoichiometry relative to the bait (RAD21) protein. (c) Comparing 30 min LysC predigestion and HCl elution. Data are mean ± SD of four measured peptides in case of SMC1, SMC3 and RAD21 and of three measured peptides in case of STAG1 and STAG2, respectively. Measurements of cohesin peptides were performed in duplicate. (d) Calculation of complex stoichiometry relative to the bait (RAD21) protein.
Figure 5
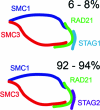
Relative abundance of STAG isoforms in the population of cohesin complexes. Cohesin is predicted to form a ring-like structure (ref (28)) with a subunit stoichiometry of 1:1:1:1 as measured in this study. The relative abundance of STAG1 containing cohesin is 6−8% and that of STAG2 is 92−94% in soluble extract of logarithmically growing HeLa cells.
Similar articles
- Cohesin and Cdk1: an anaphase barricade.
Jones KT. Jones KT. Nat Cell Biol. 2010 Feb;12(2):106-8. doi: 10.1038/ncb0210-106. Nat Cell Biol. 2010. PMID: 20118997 - Sororin, a substrate of the anaphase-promoting complex, is required for sister chromatid cohesion in vertebrates.
Rankin S, Ayad NG, Kirschner MW. Rankin S, et al. Mol Cell. 2005 Apr 15;18(2):185-200. doi: 10.1016/j.molcel.2005.03.017. Mol Cell. 2005. PMID: 15837422 - Splitting the nucleus: what's wrong with the tripartite ring model?
Nasmyth K, Oliveira RA. Nasmyth K, et al. Cold Spring Harb Symp Quant Biol. 2010;75:375-88. doi: 10.1101/sqb.2010.75.019. Epub 2011 Jan 5. Cold Spring Harb Symp Quant Biol. 2010. PMID: 21209385 - Methods to measure ubiquitin-dependent proteolysis mediated by the anaphase-promoting complex.
Kraft C, Gmachl M, Peters JM. Kraft C, et al. Methods. 2006 Jan;38(1):39-51. doi: 10.1016/j.ymeth.2005.07.005. Methods. 2006. PMID: 16343932 Review. - Structure, function and mechanism of the anaphase promoting complex (APC/C).
Barford D. Barford D. Q Rev Biophys. 2011 May;44(2):153-90. doi: 10.1017/S0033583510000259. Epub 2010 Nov 22. Q Rev Biophys. 2011. PMID: 21092369 Review.
Cited by
- A role for sister telomere cohesion in telomere elongation by telomerase.
Houghtaling BR, Canudas S, Smith S. Houghtaling BR, et al. Cell Cycle. 2012 Jan 1;11(1):19-25. doi: 10.4161/cc.11.1.18633. Epub 2012 Jan 1. Cell Cycle. 2012. PMID: 22157096 Free PMC article. - Cohesin-SA1 deficiency drives aneuploidy and tumourigenesis in mice due to impaired replication of telomeres.
Remeseiro S, Cuadrado A, Carretero M, Martínez P, Drosopoulos WC, Cañamero M, Schildkraut CL, Blasco MA, Losada A. Remeseiro S, et al. EMBO J. 2012 May 2;31(9):2076-89. doi: 10.1038/emboj.2012.11. Epub 2012 Mar 13. EMBO J. 2012. PMID: 22415365 Free PMC article. - Chemical Acetylation of Ligands and Two-Step Digestion Protocol for Reducing Codigestion in Affinity Purification-Mass Spectrometry.
Hollenstein DM, Maurer-Granofszky M, Reiter W, Anrather D, Gossenreiter T, Babic R, Hartl N, Kraft C, Hartl M. Hollenstein DM, et al. J Proteome Res. 2023 Oct 6;22(10):3383-3391. doi: 10.1021/acs.jproteome.3c00424. Epub 2023 Sep 15. J Proteome Res. 2023. PMID: 37712406 Free PMC article. - Interallelic complementation provides functional evidence for cohesin-cohesin interactions on DNA.
Eng T, Guacci V, Koshland D. Eng T, et al. Mol Biol Cell. 2015 Nov 15;26(23):4224-35. doi: 10.1091/mbc.E15-06-0331. Epub 2015 Sep 16. Mol Biol Cell. 2015. PMID: 26378250 Free PMC article. - Loss of Tumor Suppressor STAG2 Promotes Telomere Recombination and Extends the Replicative Lifespan of Normal Human Cells.
Daniloski Z, Smith S. Daniloski Z, et al. Cancer Res. 2017 Oct 15;77(20):5530-5542. doi: 10.1158/0008-5472.CAN-17-1260. Epub 2017 Aug 17. Cancer Res. 2017. PMID: 28819029 Free PMC article.
References
- Sullivan M.; Morgan D. O. Finishing mitosis, one step at a time. Nat. Rev. Mol. Cell Biol. 2007, 8 (11), 894–903. - PubMed
- Hutchins J. R.; Toyoda Y.; Hegemann B.; Poser I.; Heriche J. K.; Sykora M. M.; Augsburg M.; Hudecz O.; Buschhorn B. A.; Bulkescher J.; Conrad C.; Comartin D.; Schleiffer A.; Sarov M.; Pozniakovsky A.; Slabicki M. M.; Schloissnig S.; Steinmacher I.; Leuschner M.; Ssykor A.; Lawo S.; Pelletier L.; Stark H.; Nasmyth K.; Ellenberg J.; Durbin R.; Buchholz F.; Mechtler K.; Hyman A. A.; Peters J. M. Systematic analysis of human protein complexes identifies chromosome segregation proteins. Science 328 (5978), 593–9. - PMC - PubMed
- Kuhner S.; van Noort V.; Betts M. J.; Leo-Macias A.; Batisse C.; Rode M.; Yamada T.; Maier T.; Bader S.; Beltran-Alvarez P.; Castano-Diez D.; Chen W. H.; Devos D.; Guell M.; Norambuena T.; Racke I.; Rybin V.; Schmidt A.; Yus E.; Aebersold R.; Herrmann R.; Bottcher B.; Frangakis A. S.; Russell R. B.; Serrano L.; Bork P.; Gavin A. C. Proteome organization in a genome-reduced bacterium. Science 2009, 326 (5957), 1235–40. - PubMed
- Gavin A. C.; Bosche M.; Krause R.; Grandi P.; Marzioch M.; Bauer A.; Schultz J.; Rick J. M.; Michon A. M.; Cruciat C. M.; Remor M.; Hofert C.; Schelder M.; Brajenovic M.; Ruffner H.; Merino A.; Klein K.; Hudak M.; Dickson D.; Rudi T.; Gnau V.; Bauch A.; Bastuck S.; Huhse B.; Leutwein C.; Heurtier M. A.; Copley R. R.; Edelmann A.; Querfurth E.; Rybin V.; Drewes G.; Raida M.; Bouwmeester T.; Bork P.; Seraphin B.; Kuster B.; Neubauer G.; Superti-Furga G. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 2002, 415 (6868), 141–7. - PubMed
- Gavin A. C.; Aloy P.; Grandi P.; Krause R.; Boesche M.; Marzioch M.; Rau C.; Jensen L. J.; Bastuck S.; Dumpelfeld B.; Edelmann A.; Heurtier M. A.; Hoffman V.; Hoefert C.; Klein K.; Hudak M.; Michon A. M.; Schelder M.; Schirle M.; Remor M.; Rudi T.; Hooper S.; Bauer A.; Bouwmeester T.; Casari G.; Drewes G.; Neubauer G.; Rick J. M.; Kuster B.; Bork P.; Russell R. B.; Superti-Furga G. Proteome survey reveals modularity of the yeast cell machinery. Nature 2006, 440 (7084), 631–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources