Cell-to-cell transmission of non-prion protein aggregates - PubMed (original) (raw)
Review
Cell-to-cell transmission of non-prion protein aggregates
Seung-Jae Lee et al. Nat Rev Neurol. 2010 Dec.
Erratum in
- Nat Rev Neurol. 2011 Jan;7(1):5
Abstract
Neurodegenerative disorders such as Alzheimer disease, Parkinson disease, frontotemporal dementia, Huntington disease and Creutzfeldt-Jakob disease (CJD) are characterized by progressive accumulation of protein aggregates in selected brain regions. Protein misfolding and templated assembly into aggregates might result from an imbalance between protein synthesis, aggregation and clearance. Although protein misfolding and aggregation occur in most neurodegenerative disorders, the concept of spreading and infectivity of aggregates in the CNS has, until now, been confined to prion diseases such as CJD and bovine spongiform encephalopathy. Emerging evidence, however, suggests that prion-like spreading, involving secreted proteins such as amyloid-β and cytosolic proteins such as tau, huntingtin and α-synuclein, can occur in other neurodegenerative disorders. The underlying molecular mechanisms and the therapeutic implications of the new data are discussed in this article.
Figures
Figure 1. Aggregate clearing activity, seed formation, and aggregate burden
The formation of misfolded/aggregated protein is an inevitable outcome of a protein's life and is normally cleared by the cellular quality control mechanisms. In situation such as aging and disease, the combined effects of accelerated production, due to elevated oxidative stress, and the reduced ability of cells to degrade damaged proteins, increse protein aggregation, Incomplete degradation of aggregated proteins may result in the production of smaller fragments that can serve as seeds for further aggregation thereby increasing the aggregate burden. Therefore, reduced function in proteing degradation systems plays a critical role in aggregate propagation in neurodegenerative diseases.
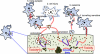
Figure 2. Models of cell-to-cell protein transmission
This diagram illustrates potential mechanisms by which misfolded/aggregated proteins are transmitted from one neuron to another. Proteins may be released from neurons via vesicle-mediated exocytosis (1) or simple leakage through damaged membranes (2), then internalize into neighboring neurons either via endocytosis or via direct penetration of the memebrane. Alternatively, as reported with the prion protein, proteins may be transmitted to the neighboring neurons by packaging into exosomes (3) or through tunneling nanotubes (4). Some or all of these mechanisms might work simultaneously, with a specific protein preferring a certain pathway to others. These mechanisms might act between the cell bodies, but could also occur trans-synaptically (5). Internalized aggregates (orange) might act as seeds for the aggregation of the endogenous native proteins (green). This seeded aggregation may produce toxic aggregate species during the course of dynamic aggregation process, which ultimately leads to the formation of pathological inclusion bodies.
Similar articles
- Protein aggregate spreading in neurodegenerative diseases: problems and perspectives.
Lee SJ, Lim HS, Masliah E, Lee HJ. Lee SJ, et al. Neurosci Res. 2011 Aug;70(4):339-48. doi: 10.1016/j.neures.2011.05.008. Epub 2011 May 20. Neurosci Res. 2011. PMID: 21624403 Free PMC article. Review. - Protein aggregation and prionopathies.
Renner M, Melki R. Renner M, et al. Pathol Biol (Paris). 2014 Jun;62(3):162-8. doi: 10.1016/j.patbio.2014.01.003. Epub 2014 Mar 31. Pathol Biol (Paris). 2014. PMID: 24698014 Review. - Interactions of amyloidogenic proteins.
Giasson BI, Lee VM, Trojanowski JQ. Giasson BI, et al. Neuromolecular Med. 2003;4(1-2):49-58. doi: 10.1385/NMM:4:1-2:49. Neuromolecular Med. 2003. PMID: 14528052 Review. - Neurodegenerative lesions: seeding and spreading.
Duyckaerts C. Duyckaerts C. Rev Neurol (Paris). 2013 Oct;169(10):825-33. doi: 10.1016/j.neurol.2013.07.018. Epub 2013 Sep 12. Rev Neurol (Paris). 2013. PMID: 24035591 Review. - A review on the cause-effect relationship between oxidative stress and toxic proteins in the pathogenesis of neurodegenerative diseases.
Borza LR. Borza LR. Rev Med Chir Soc Med Nat Iasi. 2014 Jan-Mar;118(1):19-27. Rev Med Chir Soc Med Nat Iasi. 2014. PMID: 24741770 Review.
Cited by
- Technologies and Standardization in Research on Extracellular Vesicles.
Gandham S, Su X, Wood J, Nocera AL, Alli SC, Milane L, Zimmerman A, Amiji M, Ivanov AR. Gandham S, et al. Trends Biotechnol. 2020 Oct;38(10):1066-1098. doi: 10.1016/j.tibtech.2020.05.012. Epub 2020 Jun 18. Trends Biotechnol. 2020. PMID: 32564882 Free PMC article. Review. - Epicentral disruption of structural connectivity in Alzheimer's disease.
Mallio CA, Schmidt R, de Reus MA, Vernieri F, Quintiliani L, Curcio G, Beomonte Zobel B, Quattrocchi CC, van den Heuvel MP. Mallio CA, et al. CNS Neurosci Ther. 2015 Oct;21(10):837-45. doi: 10.1111/cns.12397. Epub 2015 Apr 21. CNS Neurosci Ther. 2015. PMID: 25899584 Free PMC article. - A Protein Aggregation Inhibitor, Leuco-Methylthioninium Bis(Hydromethanesulfonate), Decreases α-Synuclein Inclusions in a Transgenic Mouse Model of Synucleinopathy.
Schwab K, Frahm S, Horsley D, Rickard JE, Melis V, Goatman EA, Magbagbeolu M, Douglas M, Leith MG, Baddeley TC, Storey JMD, Riedel G, Wischik CM, Harrington CR, Theuring F. Schwab K, et al. Front Mol Neurosci. 2018 Jan 10;10:447. doi: 10.3389/fnmol.2017.00447. eCollection 2017. Front Mol Neurosci. 2018. PMID: 29375308 Free PMC article. - Regional vulnerability in Alzheimer's disease: The role of cell-autonomous and transneuronal processes.
Acosta D, Powell F, Zhao Y, Raj A. Acosta D, et al. Alzheimers Dement. 2018 Jun;14(6):797-810. doi: 10.1016/j.jalz.2017.11.014. Epub 2018 Jan 4. Alzheimers Dement. 2018. PMID: 29306583 Free PMC article. - Polymorphic α-Synuclein Strains Modified by Dopamine and Docosahexaenoic Acid Interact Differentially with Tau Protein.
Sengupta U, Puangmalai N, Bhatt N, Garcia S, Zhao Y, Kayed R. Sengupta U, et al. Mol Neurobiol. 2020 Jun;57(6):2741-2765. doi: 10.1007/s12035-020-01913-6. Epub 2020 Apr 29. Mol Neurobiol. 2020. PMID: 32350746 Free PMC article.
References
- Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–1063. - PubMed
- Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774–779. - PubMed
- Aguzzi A, Baumann F, Bremer J. The prion's elusive reason for being. Annu Rev Neurosci. 2008;31:439–477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG11385/AG/NIA NIH HHS/United States
- R01 AG011385/AG/NIA NIH HHS/United States
- AG10435/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- P30 NS076411/NS/NINDS NIH HHS/United States
- R01 AG018440/AG/NIA NIH HHS/United States
- P01 AG031097/AG/NIA NIH HHS/United States
- AG022074/AG/NIA NIH HHS/United States
- P01 NS044233/NS/NINDS NIH HHS/United States
- R37 AG011385/AG/NIA NIH HHS/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- P01 AG010435/AG/NIA NIH HHS/United States
- AG18440/AG/NIA NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical