HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function - PubMed (original) (raw)
doi: 10.1128/JVI.02030-10. Epub 2010 Nov 3.
Boris Jülg, Augustine Pyo, Michael Flanders, Srinika Ranasinghe, Damien Z Soghoian, Douglas S Kwon, Jenna Rychert, Jeffrey Lian, Matthias I Muller, Sam Cutler, Elizabeth McAndrew, Heiko Jessen, Florencia Pereyra, Eric S Rosenberg, Marcus Altfeld, Bruce D Walker, Hendrik Streeck
Affiliations
- PMID: 21047960
- PMCID: PMC3020027
- DOI: 10.1128/JVI.02030-10
HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function
Mathieu F Chevalier et al. J Virol. 2011 Jan.
Abstract
Functional defects in cytotoxic CD8(+) T cell responses arise in chronic human viral infections, but the mechanisms involved are not well understood. In mice, CD4 cell-mediated interleukin-21 (IL-21) production is necessary for the maintenance of CD8(+) T cell function and control of persistent viral infections. To investigate the potential role of IL-21 in a chronic human viral infection, we studied the rare subset of HIV-1 controllers, who are able to spontaneously control HIV-1 replication without treatment. HIV-specific triggering of IL-21 by CD4(+) T cells was significantly enriched in these persons (P = 0.0007), while isolated loss of IL-21-secreting CD4(+) T cells was characteristic for subjects with persistent viremia and progressive disease. IL-21 responses were mediated by recognition of discrete epitopes largely in the Gag protein, and expansion of IL-21(+) CD4(+) T cells in acute infection resulted in lower viral set points (P = 0.002). Moreover, IL-21 production by CD4(+) T cells of HIV controllers enhanced perforin production by HIV-1-specific CD8(+) T cells from chronic progressors even in late stages of disease, and HIV-1-specific effector CD8(+) T cells showed an enhanced ability to efficiently inhibit viral replication in vitro after IL-21 binding. These data suggest that HIV-1-specific IL-21(+) CD4(+) T cell responses might contribute to the control of viral replication in humans and are likely to be of great importance for vaccine design.
Figures
FIG. 1.
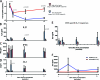
IL-21 production by CD4+ T cells in HIV-1-infected subjects. (A and B) CD8-depleted PBMCs from HIV-1 chronic progressors, ART-treated patients, HIV-1 controllers, and HIV-negative subjects were stimulated with PMA-ionomycin (A) or with HIV Gag peptide pool or the CMV peptide pp65 (B) for 40 h at 37°C in the bottom chamber of a transwell plate with anti-IL-21 Luminex beads coincubated in the upper chamber. Beads were then used for the quantification of secreted IL-21. Mann-Whitney t tests were performed; only significant P values are indicated. Significantly higher IL-21 responses were detected in the controllers than in the other groups. HIV-specific IL-21+ responses were directed against epitopes within the Gag protein in HIV-1 controllers, whereas rare specific responses were detected in the progressors. Error bars indicate standard deviations. (C) Analysis of the secretion of IL-21 and IFN-γ in five HIV-1 controllers following stimulation with individual peptides spanning the HIV Gag proteins p17, p24, and p15. The color intensity indicates the relative magnitude of the IFN-γ response and the IL-21 response to each peptide.
FIG. 2.
IL-21+ CD4+ T cell responses in acute HIV infection. (A) Twelve individuals with acute HIV infection were grouped based on their viral load set points at 12 month after presentation (high, 55,900 to 1,600,000 RNA copies/ml; low, 4,810 to 39,400 RNA copies/ml). PBMCs from these individuals were obtained at baseline and 2, 6, and 12 months after initial presentation. (B to D) PBMCs were CD8 depleted and stimulated with Gag peptide pool, and IL-21 (B), IL-2 (C), and IFN-γ (D) secretion was measured by Luminex. (E) Following the same protocol, CMV-specific IL-21 secretion was assessed at the same time points in all individuals. (F) Longitudinal assessment of IL-21 production by Gag-stimulated CD4+ T cells in treated (n = 4) and untreated (n = 4) subjects during acute HIV-1 infection, showing an initial increase in the early phase of acute infection in treated subjects which then decreased. Error bars indicate standard deviations.
FIG. 3.
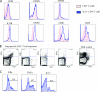
Phenotypic and functional characteristics of HIV-1-specific IL-21+ CD4+ T cells. (A and B) Phenotypic (A) and functional (B) characterization of HIV-1-specific IL-21+ CD4+ T cells (representative for six controllers). For assessing intracellular cytokine secretion, cells of four HIV controllers were stimulated as indicated with the Gag peptide pool or SEB for 5 h in IL-21-supplemented medium (50 ng/ml). (C) Functional characterization of IL-21+ CD4+ T cells after SEB stimulation.
FIG. 4.
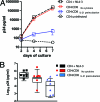
CD8+ T cells responses to IL-21 stimulation. (A) PBMCs from eight HIV-1-infected progressors and nine controllers were cultured for 5 h in the presence or absence of HIV CD8+ optimal peptides and IL-21 (50 ng/ml). Box plots represent the IL-21R expression on antigen-specific (tetramer-positive) CD8+ T cells detected by flow cytometry. (B) PBMCs of 16 HIV-infected progressors (black) and 5 HIV controllers (green) were cultured with or without IL-21, and the percentage of CD8+ T cells expressing CD107a, IFN-γ, or TNF-α was assessed by flow-cytometry using intracellular cytokine staining. (C) Granzyme A, granzyme B, and perforin mRNA upregulation measured by quantitative PCR on bulk CD8+ T cells after IL-21 stimulation of PBMCs from 10 untreated HIV-infected subjects. (D) IL-21-mediated perforin upregulation in tetramer-positive CD8+ T cells of 17 untreated HIV-infected subjects was measured by flow cytometry. These results together demonstrate the ability of IL-21 to increase both the bulk and HIV-specific CD8+ T cell cytotoxic potential of chronic progressors. (E) CD8-depleted PBMCs from six controllers were prestimulated with Gag peptides for 1 h or left unstimulated. The cells were thoroughly washed and coincubated for 12 h in the bottom chamber of a transwell plate with CD8+ T cells from a chronic progressor in the upper chamber. IL-21 blocking was performed by adding neutralizing anti-IL-21 and anti-IL-21 receptor antibodies to the CD8+ T cells in the upper chamber. After the incubation, CD8+ T cells were harvested and perforin mRNA relative levels were assessed by quantitative PCR (bar diagrams). Error bars indicate standard deviations.
FIG. 5.
IL-21 induces suppression of HIV-1 replication by bulk CD8+ T cells. In order to test whether IL-21 can improve the viral suppressive capacity of bulk CD8+ T cells, we selected subjects with a known lack of CD8+ T cell viral inhibition (assessed previously) to understand the impact of IL-21 on their inhibitory capacity. (A) Representative results from one individual. Bulk CD8+ T cells, isolated from frozen PBMCs by positive selection and rested for 2 days, failed to inhibit replication of HIV-1 virus NL4-3 (X4 tropic) in autologous CD4+ T cells at a CD8-to-CD4 T cell ratio of 1:1. However, preincubation of the CD8 T cells with IL-21 (50 ng/ml) led to a 1000 fold viral inhibition. (B) Comparison of the viral suppressive capacities of bulk CD8+ T cells with or without preincubation with IL-21 in seven subjects selected based on a previous assessment of their inhibitory capacity. First we confirmed that in the presence of CD8+ T cells, viral replication was not significantly reduced (log10 p24 [pg/ml], 5.18 ± 0.36 versus 4.63 ± 0.76 for CD4+ controls and CD4-CD8 cocultures, respectively [n.s.]). However, preincubation of the CD8+ T cells with IL-21 improved the inhibitory effect significantly (log10 p24 [pg/ml], 3.71 ± 1.11 [P < 0.05]). Error bars indicate standard deviations.
Similar articles
- Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers.
Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J, Zajac AJ, Goepfert PA. Williams LD, et al. J Virol. 2011 Mar;85(5):2316-24. doi: 10.1128/JVI.01476-10. Epub 2010 Dec 15. J Virol. 2011. PMID: 21159862 Free PMC article. - Comparative analysis of the capacity of elite suppressor CD4+ and CD8+ T cells to inhibit HIV-1 replication in monocyte-derived macrophages.
Walker-Sperling VE, Buckheit RW 3rd, Blankson JN. Walker-Sperling VE, et al. J Virol. 2014 Sep 1;88(17):9789-98. doi: 10.1128/JVI.00860-14. Epub 2014 Jun 18. J Virol. 2014. PMID: 24942573 Free PMC article. - HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control.
Yue FY, Lo C, Sakhdari A, Lee EY, Kovacs CM, Benko E, Liu J, Song H, Jones RB, Sheth P, Chege D, Kaul R, Ostrowski MA. Yue FY, et al. J Immunol. 2010 Jul 1;185(1):498-506. doi: 10.4049/jimmunol.0903915. Epub 2010 Jun 2. J Immunol. 2010. PMID: 20519650 - HIV-specific CD4 T cells and immune control of viral replication.
Porichis F, Kaufmann DE. Porichis F, et al. Curr Opin HIV AIDS. 2011 May;6(3):174-80. doi: 10.1097/COH.0b013e3283454058. Curr Opin HIV AIDS. 2011. PMID: 21502921 Free PMC article. Review. - The role of interleukin-21 in HIV infection.
Pallikkuth S, Parmigiani A, Pahwa S. Pallikkuth S, et al. Cytokine Growth Factor Rev. 2012 Aug-Oct;23(4-5):173-80. doi: 10.1016/j.cytogfr.2012.05.004. Epub 2012 Jul 2. Cytokine Growth Factor Rev. 2012. PMID: 22763176 Free PMC article. Review.
Cited by
- Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection.
Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. Aubert RD, et al. Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):21182-7. doi: 10.1073/pnas.1118450109. Epub 2011 Dec 12. Proc Natl Acad Sci U S A. 2011. PMID: 22160724 Free PMC article. - A Phase I Double Blind, Placebo-Controlled, Randomized Study of the Safety and Immunogenicity of an Adjuvanted HIV-1 Gag-Pol-Nef Fusion Protein and Adenovirus 35 Gag-RT-Int-Nef Vaccine in Healthy HIV-Uninfected African Adults.
Omosa-Manyonyi G, Mpendo J, Ruzagira E, Kilembe W, Chomba E, Roman F, Bourguignon P, Koutsoukos M, Collard A, Voss G, Laufer D, Stevens G, Hayes P, Clark L, Cormier E, Dally L, Barin B, Ackland J, Syvertsen K, Zachariah D, Anas K, Sayeed E, Lombardo A, Gilmour J, Cox J, Fast P, Priddy F. Omosa-Manyonyi G, et al. PLoS One. 2015 May 11;10(5):e0125954. doi: 10.1371/journal.pone.0125954. eCollection 2015. PLoS One. 2015. PMID: 25961283 Free PMC article. Clinical Trial. - HIV controllers maintain a population of highly efficient Th1 effector cells in contrast to patients treated in the long term.
Vingert B, Benati D, Lambotte O, de Truchis P, Slama L, Jeannin P, Galperin M, Perez-Patrigeon S, Boufassa F, Kwok WW, Lemaître F, Delfraissy JF, Thèze J, Chakrabarti LA. Vingert B, et al. J Virol. 2012 Oct;86(19):10661-74. doi: 10.1128/JVI.00056-12. Epub 2012 Jul 25. J Virol. 2012. PMID: 22837194 Free PMC article. - Augmentation of HIV-specific T cell function by immediate treatment of hyperacute HIV-1 infection.
Ndhlovu ZM, Kazer SW, Nkosi T, Ogunshola F, Muema DM, Anmole G, Swann SA, Moodley A, Dong K, Reddy T, Brockman MA, Shalek AK, Ndung'u T, Walker BD. Ndhlovu ZM, et al. Sci Transl Med. 2019 May 22;11(493):eaau0528. doi: 10.1126/scitranslmed.aau0528. Sci Transl Med. 2019. PMID: 31118290 Free PMC article. Clinical Trial. - Increased memory differentiation is associated with decreased polyfunctionality for HIV but not for cytomegalovirus-specific CD8+ T cells.
Riou C, Treurnicht F, Abrahams MR, Mlisana K, Liu MK, Goonetilleke N, Koup R, Roederer M, Abdool Karim S, de Bruyn G, Williamson C, Gray CM, Burgers WA; CAPRISA 002 Study Team. Riou C, et al. J Immunol. 2012 Oct 15;189(8):3838-47. doi: 10.4049/jimmunol.1201488. Epub 2012 Sep 10. J Immunol. 2012. PMID: 22966086 Free PMC article.
References
- Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. - PubMed
- Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. - PubMed
- Frohlich, A., J. Kisielow, I. Schmitz, S. Freigang, A. T. Shamshiev, J. Weber, B. J. Marsland, A. Oxenius, and M. Kopf. 2009. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324:1576-1580. - PubMed
- Gosselin, A., P. Monteiro, N. Chomont, F. Diaz-Griffero, E. A. Said, S. Fonseca, V. Wacleche, M. El-Far, M. R. Boulassel, J. P. Routy, R. P. Sekaly, and P. Ancuta. 2010. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J. Immunol. 184:1604-1616. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007387/AI/NIAID NIH HHS/United States
- R01 AI091450/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AI091450-01/AI/NIAID NIH HHS/United States
- K08 AI084546/AI/NIAID NIH HHS/United States
- R01 AI091450-02/AI/NIAID NIH HHS/United States
- 1R01AI091450-01/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- R01 AI030914/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials