Safety and immunogenicity of a replication-defective adenovirus type 5 HIV vaccine in Ad5-seronegative persons: a randomized clinical trial (HVTN 054) - PubMed (original) (raw)
Randomized Controlled Trial
Safety and immunogenicity of a replication-defective adenovirus type 5 HIV vaccine in Ad5-seronegative persons: a randomized clinical trial (HVTN 054)
Laurence Peiperl et al. PLoS One. 2010.
Abstract
Background: Individuals without prior immunity to a vaccine vector may be more sensitive to reactions following injection, but may also show optimal immune responses to vaccine antigens. To assess safety and maximal tolerated dose of an adenoviral vaccine vector in volunteers without prior immunity, we evaluated a recombinant replication-defective adenovirus type 5 (rAd5) vaccine expressing HIV-1 Gag, Pol, and multiclade Env proteins, VRC-HIVADV014-00-VP, in a randomized, double-blind, dose-escalation, multicenter trial (HVTN study 054) in HIV-1-seronegative participants without detectable neutralizing antibodies (nAb) to the vector. As secondary outcomes, we also assessed T-cell and antibody responses.
Methodology/principal findings: Volunteers received one dose of vaccine at either 10(10) or 10(11) adenovector particle units, or placebo. T-cell responses were measured against pools of global potential T-cell epitope peptides. HIV-1 binding and neutralizing antibodies were assessed. Systemic reactogenicity was greater at the higher dose, but the vaccine was well tolerated at both doses. Although no HIV infections occurred, commercial diagnostic assays were positive in 87% of vaccinees one year after vaccination. More than 85% of vaccinees developed HIV-1-specific T-cell responses detected by IFN-γ ELISpot and ICS assays at day 28. T-cell responses were: CD8-biased; evenly distributed across the three HIV-1 antigens; not substantially increased at the higher dose; and detected at similar frequencies one year following injection. The vaccine induced binding antibodies against at least one HIV-1 Env antigen in all recipients.
Conclusions/significance: This vaccine appeared safe and was highly immunogenic following a single dose in human volunteers without prior nAb against the vector.
Trial registration: ClinicalTrials.gov NCT00119873.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
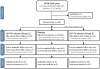
Figure 1. CONSORT statement 2010 flow diagram.
Precise enrollment screening numbers are not available due to the candidate pool being screened for eligibility in multiple HVTN trials. Group 2 intervention proceeded only after a safety review of Group 1 interventions. For all analyses, both placebo groups were combined into one pool. Numbers shown in the analysis row are maximum available, with some specific assays using smaller numbers due to assay-specific losses, as detailed in the results.
Figure 2. Duration and intensity of systemic reactogenicity.
Number of vaccine recipients experiencing each level of severity in one or more systemic symptoms (malaise and/or fatigue, myalgia, headache, nausea, vomiting, chills, or arthralgia) is shown at baseline and for each day of the reactogenicity period. Panel A: 1010 PU dose; Panel B: 1011 PU dose. Only the most severe symptom level for each individual on each day is included.
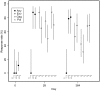
Figure 3. T-cell responses to HIV antigens by IFN-γ ELISpot.
Points indicate percentage of participants with positive responses; n denotes the number in each group with ELISpot data. Lines indicate 95% confidence intervals.
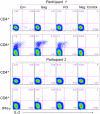
Figure 4. Staining profiles for ICS analysis for two trial participants.
The expression of IFN-γ and IL-2 are shown for CD4+ and CD8+ T cells in response to stimulation with the first pools for Env, Gag, and Pol and for the negative control (peptide diluent, 1% DMSO) at day 28. Numbers on the plots are the percentages of CD4+ or CD8+ T cells producing the cytokine or combination of cytokines. Participant 1 represents an example of a high CD8+ T-cell response and Participant 2 represents low CD4+ and CD8+ T-cell responses. All responses are positive as tested for cells producing IFN-γ and/or IL-2 except for Env and Pol for participant 2. Both participants were in the 1011 PU dose group.
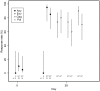
Figure 5. T-cell responses to HIV antigens by ICS.
Points indicate percentage of participants with positive responses (for CD4+ or CD8+ T cell subsets, and for IFN-γ or IL-2 detection); n denotes the number in each group with ICS data. Lines indicate 95% confidence intervals.
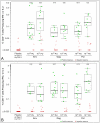
Figure 6. Magnitudes of T-cell responses to pool 1 global PTE peptides, measured by IFN-γ ELISpot assay.
Day 0 (left column) is a combined analysis including responses from PBMC from placebo recipients; day 28 (remaining columns) is sorted by HIV-1 gene product. The box plots are based only on the positive responses. The box indicates the median and interquartile range (IQR); whiskers extend to the furthest point within 1.5 times the IQR from the upper or lower quartile. The number of vaccinees with a positive response out of the total number of vaccinees tested is indicated above the bars.
Figure 7. Magnitudes of responses by ICS to pool 1 peptides at day 28 following study injection.
A) CD4+ T cell responses. B) CD8+ T cell responses. The box plots are based only on the positive responses. The box indicates the median and interquartile range (IQR); whiskers extend to the furthest point within 1.5 times the IQR from the upper or lower quartile. The number of vaccinees with a positive response out of the total number of vaccinees tested is indicated above the bars.
Similar articles
- Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa.
Jaoko W, Karita E, Kayitenkore K, Omosa-Manyonyi G, Allen S, Than S, Adams EM, Graham BS, Koup RA, Bailer RT, Smith C, Dally L, Farah B, Anzala O, Muvunyi CM, Bizimana J, Tarragona-Fiol T, Bergin PJ, Hayes P, Ho M, Loughran K, Komaroff W, Stevens G, Thomson H, Boaz MJ, Cox JH, Schmidt C, Gilmour J, Nabel GJ, Fast P, Bwayo J. Jaoko W, et al. PLoS One. 2010 Sep 21;5(9):e12873. doi: 10.1371/journal.pone.0012873. PLoS One. 2010. PMID: 20877623 Free PMC article. Clinical Trial. - A phase 1/2 study of a multiclade HIV-1 DNA plasmid prime and recombinant adenovirus serotype 5 boost vaccine in HIV-Uninfected East Africans (RV 172).
Kibuuka H, Kimutai R, Maboko L, Sawe F, Schunk MS, Kroidl A, Shaffer D, Eller LA, Kibaya R, Eller MA, Schindler KB, Schuetz A, Millard M, Kroll J, Dally L, Hoelscher M, Bailer R, Cox JH, Marovich M, Birx DL, Graham BS, Michael NL, de Souza MS, Robb ML. Kibuuka H, et al. J Infect Dis. 2010 Feb 15;201(4):600-7. doi: 10.1086/650299. J Infect Dis. 2010. PMID: 20078213 Free PMC article. Clinical Trial. - Safety and Immunogenicity of a rAd35-EnvA Prototype HIV-1 Vaccine in Combination with rAd5-EnvA in Healthy Adults (VRC 012).
Crank MC, Wilson EM, Novik L, Enama ME, Hendel CS, Gu W, Nason MC, Bailer RT, Nabel GJ, McDermott AB, Mascola JR, Koup RA, Ledgerwood JE, Graham BS; VRC012 Study Team. Crank MC, et al. PLoS One. 2016 Nov 15;11(11):e0166393. doi: 10.1371/journal.pone.0166393. eCollection 2016. PLoS One. 2016. PMID: 27846256 Free PMC article. Clinical Trial. - Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector.
Catanzaro AT, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, Gu L, Martin JE, Novik L, Chakrabarti BK, Butman BT, Gall JG, King CR, Andrews CA, Sheets R, Gomez PL, Mascola JR, Nabel GJ, Graham BS; Vaccine Research Center 006 Study Team. Catanzaro AT, et al. J Infect Dis. 2006 Dec 15;194(12):1638-49. doi: 10.1086/509258. Epub 2006 Nov 8. J Infect Dis. 2006. PMID: 17109335 Free PMC article. Clinical Trial. - A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204).
Churchyard GJ, Morgan C, Adams E, Hural J, Graham BS, Moodie Z, Grove D, Gray G, Bekker LG, McElrath MJ, Tomaras GD, Goepfert P, Kalams S, Baden LR, Lally M, Dolin R, Blattner W, Kalichman A, Figueroa JP, Pape J, Schechter M, Defawe O, De Rosa SC, Montefiori DC, Nabel GJ, Corey L, Keefer MC; NIAID HIV Vaccine Trials Network. Churchyard GJ, et al. PLoS One. 2011;6(8):e21225. doi: 10.1371/journal.pone.0021225. Epub 2011 Aug 3. PLoS One. 2011. PMID: 21857901 Free PMC article. Clinical Trial.
Cited by
- Viral Vectors for the Induction of Broadly Neutralizing Antibodies against HIV.
Wilmschen S, Schmitz JE, Kimpel J. Wilmschen S, et al. Vaccines (Basel). 2019 Sep 19;7(3):119. doi: 10.3390/vaccines7030119. Vaccines (Basel). 2019. PMID: 31546894 Free PMC article. Review. - Development of a Minor Histocompatibility Antigen Vaccine Regimen in the Canine Model of Hematopoietic Cell Transplantation.
Rosinski SL, Stone B, Graves SS, Fuller DH, De Rosa SC, Spies GA, Mize GJ, Fuller JT, Storb R. Rosinski SL, et al. Transplantation. 2015 Oct;99(10):2083-94. doi: 10.1097/TP.0000000000000744. Transplantation. 2015. PMID: 25965411 Free PMC article. - HIV-1-Specific Antibody Response and Function after DNA Prime and Recombinant Adenovirus 5 Boost HIV Vaccine in HIV-Infected Subjects.
Gach JS, Gorlani A, Dotsey EY, Becerra JC, Anderson CT, Berzins B, Felgner PL, Forthal DN, Deeks SG, Wilkin TJ, Casazza JP, Koup RA, Katlama C, Autran B, Murphy RL, Achenbach CJ. Gach JS, et al. PLoS One. 2016 Aug 8;11(8):e0160341. doi: 10.1371/journal.pone.0160341. eCollection 2016. PLoS One. 2016. PMID: 27500639 Free PMC article. Clinical Trial. - A Recombinant Adenovirus Encoding Multiple HIV-1 Epitopes Induces Stronger CD4+ T cell Responses than a DNA Vaccine in Mice.
Rosa DS, Ribeiro SP, Almeida RR, Mairena EC, Kalil J, Cunha-Neto E. Rosa DS, et al. J Vaccines Vaccin. 2011 Dec 2;2(4):1000124. doi: 10.4172/2157-7560.1000124. J Vaccines Vaccin. 2011. PMID: 23814696 Free PMC article. - Safety and immunogenicity of an HIV adenoviral vector boost after DNA plasmid vaccine prime by route of administration: a randomized clinical trial.
Koblin BA, Casapia M, Morgan C, Qin L, Wang ZM, Defawe OD, Baden L, Goepfert P, Tomaras GD, Montefiori DC, McElrath MJ, Saavedra L, Lau CY, Graham BS; NIAID HIV Vaccine Trials Network. Koblin BA, et al. PLoS One. 2011;6(9):e24517. doi: 10.1371/journal.pone.0024517. Epub 2011 Sep 12. PLoS One. 2011. PMID: 21931737 Free PMC article. Clinical Trial.
References
- UNAIDS. 2007. AIDS Epidemic Update 2007. Available: http://www.unaids.org/
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. - PubMed
- Robinson HL, Montefiori DC, Johnson RP, Manson KH, Kalish ML, et al. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med. 1999;5:526–534. - PubMed
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials