Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit - PubMed (original) (raw)
Review
Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit
Hitoshi Ishimoto et al. Endocr Rev. 2011 Jun.
Abstract
Continuous efforts have been devoted to unraveling the biophysiology and development of the human fetal adrenal cortex, which is structurally and functionally unique from other species. It plays a pivotal role, mainly through steroidogenesis, in the regulation of intrauterine homeostasis and in fetal development and maturation. The steroidogenic activity is characterized by early transient cortisol biosynthesis, followed by its suppressed synthesis until late gestation, and extensive production of dehydroepiandrosterone and its sulfate, precursors of placental estrogen, during most of gestation. The gland rapidly grows through processes including cell proliferation and angiogenesis at the gland periphery, cellular migration, hypertrophy, and apoptosis. Recent studies employing modern technologies such as gene expression profiling and laser capture microdissection have revealed that development and/or function of the fetal adrenal cortex may be regulated by a panoply of molecules, including transcription factors, extracellular matrix components, locally produced growth factors, and placenta-derived CRH, in addition to the primary regulator, fetal pituitary ACTH. The role of the fetal adrenal cortex in human pregnancy and parturition appears highly complex, probably due to redundant and compensatory mechanisms regulating these events. Mounting evidence indicates that actions of hormones operating in the human feto-placental unit are likely mediated by mechanisms including target tissue responsiveness, local metabolism, and bioavailability, rather than changes only in circulating levels. Comprehensive study of such molecular mechanisms and the newly identified factors implicated in adrenal development should help crystallize our understanding of the development and physiology of the human fetal adrenal cortex.
Figures
Fig. 1.
Hypothetical model of development of the midgestation HFA gland. The schema shows the HFA cortex structure, which consists of the DZ, TZ, and FZ. The medulla is not recognized as a distinct structure in the HFA throughout most of gestation. Previous studies from our laboratory and others have demonstrated that the HFA cortex is a dynamic organ: hyperplasia occurs primarily in the DZ; hypertrophy occurs primarily in the FZ; and apoptosis occurs primarily in the central portion of the FZ. Thus, cells appear to proliferate in the periphery, migrate centripetally, differentiate to form the specialized cortical compartments, and then undergo senescence when they reach the center of the cortex. Recent studies indicate that angiogenesis (formation of new capillaries from preexisting blood vessels) occurs at the periphery of the gland. C, Capsule.
Fig. 2.
Principal pathways of human steroid biosynthesis. Pathways for biosynthesis of progesterone, mineralocorticoids, glucocorticoids, androgens, and estrogens. Names of the enzymes that catalyze each step are indicated in boxes. 17-OH Preg, 17-Hydroxypregnenolone; 17-OH Prog, 17-hydroxyprogesterone. *, 11-Deoxycorticosterone is converted to corticosterone by 11-hydroxylase activity of CYP11B2 (in zona glomerulosa) or by CYP11B1 (in zona fasciculata). #, The conversion of 17-hydroxyprogesterone to androstenedione by CYP17 is limited in humans.
Fig. 3.
Zonal expression of steroidogenic components in the HFA cortex during mid- and late gestation. Schema shows the specific zonal expression of components of the steroidogenic machinery. Black crossbar denotes expression, whereas absence of a bar represents lack of expression. Gray depicts low levels of expression. Figure is derived from previous studies (7, 11, 64, 70).
Fig. 4.
Zonal expression of putative factors implicated in HFA development and/or function. Schema shows specific zonal expression of putative factors implicated in HFA development and/or function. Black crossbar denotes high levels of expression, whereas absence of a bar represents lack of expression. Dark gray depicts moderate levels of expression, and light gray depicts low levels. LDL-R, LDL receptor; PCNA, proliferating cell nuclear antigen. Figure was derived from studies shown in right column.
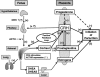
Fig. 5.
Schematic representation of hypothetical model describing endocrine cascades in human feto-placental unit. Near term, placenta-derived CRH increases and directly stimulates HFA production of DHEA/DHEAS and cortisol. Increased cortisol, in turn, stimulates production of placental CRH. CRH also up-regulates expression of the ACTHR, thereby increasing HFA responsiveness to ACTH, the level in fetal circulation of which may be constant or decrease near term. HFA and placental CRH constitute positive feedback cascade. DHEA/DHEAS is converted by placenta to estrogen, promoting initiation of parturition partly through up-regulation of contraction-associated proteins. Cortisol also promotes maturation of fetal organs (e.g., the lung) and stimulates production of prostaglandins, uterotonins necessary for parturitional processes. CRH also directly promotes initiation of parturition in part by increasing prostaglandins. When “functional withdrawal” of progesterone occurs, coupled with these changes, parturition is initiated.
Similar articles
- Developmental and functional biology of the primate fetal adrenal cortex.
Mesiano S, Jaffe RB. Mesiano S, et al. Endocr Rev. 1997 Jun;18(3):378-403. doi: 10.1210/edrv.18.3.0304. Endocr Rev. 1997. PMID: 9183569 Review. - Actions of placental and fetal adrenal steroid hormones in primate pregnancy.
Pepe GJ, Albrecht ED. Pepe GJ, et al. Endocr Rev. 1995 Oct;16(5):608-48. doi: 10.1210/edrv-16-5-608. Endocr Rev. 1995. PMID: 8529574 Review. - Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum.
Mastorakos G, Ilias I. Mastorakos G, et al. Ann N Y Acad Sci. 2003 Nov;997:136-49. doi: 10.1196/annals.1290.016. Ann N Y Acad Sci. 2003. PMID: 14644820 Review. - Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells.
Smith R, Mesiano S, Chan EC, Brown S, Jaffe RB. Smith R, et al. J Clin Endocrinol Metab. 1998 Aug;83(8):2916-20. doi: 10.1210/jcem.83.8.5020. J Clin Endocrinol Metab. 1998. PMID: 9709969 - Role of growth factors in the developmental regulation of the human fetal adrenal cortex.
Mesiano S, Jaffe RB. Mesiano S, et al. Steroids. 1997 Jan;62(1):62-72. doi: 10.1016/s0039-128x(96)00161-4. Steroids. 1997. PMID: 9029717 Review.
Cited by
- Evaluating the role of aldosterone synthesis on adrenal cell fate.
Aminuddin A, Brown MJ, Azizan EA. Aminuddin A, et al. Front Endocrinol (Lausanne). 2024 Aug 7;15:1423027. doi: 10.3389/fendo.2024.1423027. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39170743 Free PMC article. Review. - Sexual Dimorphism of Corticosteroid Signaling during Kidney Development.
Laulhé M, Dumeige L, Vu TA, Hani I, Pussard E, Lombès M, Viengchareun S, Martinerie L. Laulhé M, et al. Int J Mol Sci. 2021 May 18;22(10):5275. doi: 10.3390/ijms22105275. Int J Mol Sci. 2021. PMID: 34069759 Free PMC article. Review. - Current insight into the transient X-zone in the adrenal gland cortex.
Kang Y, Laprocina K, Zheng HS, Huang CJ. Kang Y, et al. Vitam Horm. 2024;124:297-339. doi: 10.1016/bs.vh.2023.05.003. Epub 2023 Jul 12. Vitam Horm. 2024. PMID: 38408801 Free PMC article. Review. - Maternal Exposure to Childhood Trauma Is Associated During Pregnancy With Placental-Fetal Stress Physiology.
Moog NK, Buss C, Entringer S, Shahbaba B, Gillen DL, Hobel CJ, Wadhwa PD. Moog NK, et al. Biol Psychiatry. 2016 May 15;79(10):831-839. doi: 10.1016/j.biopsych.2015.08.032. Epub 2015 Sep 3. Biol Psychiatry. 2016. PMID: 26444076 Free PMC article. - Premature Birth and Developmental Programming: Mechanisms of Resilience and Vulnerability.
Lammertink F, Vinkers CH, Tataranno ML, Benders MJNL. Lammertink F, et al. Front Psychiatry. 2021 Jan 8;11:531571. doi: 10.3389/fpsyt.2020.531571. eCollection 2020. Front Psychiatry. 2021. PMID: 33488409 Free PMC article. Review.
References
- Mesiano S, Jaffe RB. 1997. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev 18:378–403 - PubMed
- Diczfalusy E. 1964. Endocrine functions of the human fetoplacental unit. Fed Proc 23:791–798 - PubMed
- Liggins GC, Fairclough RJ, Grieves SA, Kendall JZ, Knox BS. 1973. The mechanism of initiation of parturition in the ewe. Recent Prog Horm Res 29:111–159 - PubMed
- Liggins GC. 1974. Parturition in the sheep and the human. Basic Life Sci 4:423–443 - PubMed
- Jirasek JE. 1980. Human fetal endocrines. London: Martinus Nijhoff; 69–82
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical