Specification and morphogenesis of astrocytes - PubMed (original) (raw)
Review
Specification and morphogenesis of astrocytes
Marc R Freeman. Science. 2010.
Abstract
Astrocytes are the most abundant cell type in the mammalian brain. Interest in astrocyte function has increased dramatically in recent years because of their newly discovered roles in synapse formation, maturation, efficacy, and plasticity. However, our understanding of astrocyte development has lagged behind that of other brain cell types. We do not know the molecular mechanism by which astrocytes are specified, how they grow to assume their complex morphologies, and how they interact with and sculpt developing neuronal circuits. Recent work has provided a basic understanding of how intrinsic and extrinsic mechanisms govern the production of astrocytes from precursor cells and the generation of astrocyte diversity. Moreover, new studies of astrocyte morphology have revealed that mature astrocytes are extraordinarily complex, interact with many thousands of synapses, and tile with other astrocytes to occupy unique spatial domains in the brain. A major challenge for the field is to understand how astrocytes talk to each other, and to neurons, during development to establish appropriate astrocytic and neuronal network architectures.
Figures
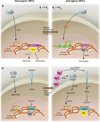
Fig. 1
Intrinsic epigenetic mechanisms converge with extrinsic signals to promote astrocyte fate from NPCs. (A) In early NPCs, chromatin is open at the neurogenin1 and neurogenin2 (ngn1 and 2) loci, Wnt signals can activate their expression, and neurogenins promote neuronal fate and inhibit astrocyte fate. In postnatal NPCs, the ngn1 and 2 loci have been methylated and bound by PcG, chromatin is closed, Wnt-dependent ngn1 and 2 expression is suppressed, and astrocyte fate is in turn derepressed. (B) Astrocyte genes are methylated during embryonic stages, which closes local chromatin; this is maintained by DNMT1, and astrocyte fate is suppressed, despite the presence of low levels of the proastrocyte factor CT-1. At the switch from NPC production of neurons to astrocytes, Ngn+ neurons release additional CT-1, which activates JAK-STAT signaling, and also activates Notch signaling (likely through JAG1 or DLL2), which activates NFIA, an inhibitor of Dnmt1 activity. Positive JAK-STAT signaling, coupled with opening of chromatin at astrocyte gene loci, promotes astrocyte fate.
Fig. 2
Astrocyte diversity is generated by production from unique spatial domains. (A) A schematic illustrating VA1, VA2, and VA3 domains of the spinal cord white matter astrocytes and expression domains of Reelin and Slit1. (B) A homeodomain code that controls the generation of neuronal diversity is reused during astrocyte specification to promote white matter astrocyte diversity. [(A) and (B) are adapted from (12)]
Fig. 3
Astrocyte morphology is far more complex than initially appreciated. Astrocytes in an organotypic slice preparation labeled with the common marker GFAP (red) overlaid with a single astrocyte transfected with the membrane marker Lck-GFP (green fluorescent protein). Arrow indicates GFAP label of Lck-GFP–marked astrocyte. [From (53) with permission from Elsevier]
Fig. 4
Coordination of astrocyte morphological growth and refinement with synapse formation. (A) Although most neurons are made during embryonic stages, the major waves of synaptogenesis follow and depend on astrocyte production. The timing of astrocyte growth and morphological refinement overlaps significantly with this window of synaptogenesis. (B) Astrocytes initially extend large, filopodial processes that overlap significantly with neighboring astrocytes; however, by postnatal day 21, astrocytes refine their morphology to occupy unique spatial domains and elaborate fine processes that closely associate with synapses. (C) A complex interrelation exists between synapses and astrocyte processes. Eph or ephrin signaling can bidirectionally control astrocyte features, as well as spine morphology (see text).
Similar articles
- Live-imaging of astrocyte morphogenesis and function in zebrafish neural circuits.
Chen J, Poskanzer KE, Freeman MR, Monk KR. Chen J, et al. Nat Neurosci. 2020 Oct;23(10):1297-1306. doi: 10.1038/s41593-020-0703-x. Epub 2020 Sep 7. Nat Neurosci. 2020. PMID: 32895565 Free PMC article. - Astrocyte lineage cells are essential for functional neuronal differentiation and synapse maturation in human iPSC-derived neural networks.
Klapper SD, Garg P, Dagar S, Lenk K, Gottmann K, Nieweg K. Klapper SD, et al. Glia. 2019 Oct;67(10):1893-1909. doi: 10.1002/glia.23666. Epub 2019 Jun 27. Glia. 2019. PMID: 31246351 - Emerging mechanisms underlying astrogenesis in the developing mammalian brain.
Takouda J, Katada S, Nakashima K. Takouda J, et al. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(6):386-398. doi: 10.2183/pjab.93.024. Proc Jpn Acad Ser B Phys Biol Sci. 2017. PMID: 28603210 Free PMC article. Review. - A star is born: new insights into the mechanism of astrogenesis.
Kanski R, van Strien ME, van Tijn P, Hol EM. Kanski R, et al. Cell Mol Life Sci. 2014 Feb;71(3):433-47. doi: 10.1007/s00018-013-1435-9. Epub 2013 Aug 2. Cell Mol Life Sci. 2014. PMID: 23907612 Free PMC article. Review. - Dynamism of an Astrocyte In Vivo: Perspectives on Identity and Function.
Poskanzer KE, Molofsky AV. Poskanzer KE, et al. Annu Rev Physiol. 2018 Feb 10;80:143-157. doi: 10.1146/annurev-physiol-021317-121125. Epub 2017 Nov 20. Annu Rev Physiol. 2018. PMID: 29166242 Free PMC article. Review.
Cited by
- Astrocyte regional specialization is shaped by postnatal development.
Schroeder ME, McCormack DM, Metzner L, Kang J, Li KX, Yu E, Levandowski KM, Zaniewski H, Zhang Q, Boyden ES, Krienen FM, Feng G. Schroeder ME, et al. bioRxiv [Preprint]. 2024 Oct 26:2024.10.11.617802. doi: 10.1101/2024.10.11.617802. bioRxiv. 2024. PMID: 39416060 Free PMC article. Preprint. - Extracellular Vesicle-Mediated Neuron-Glia Communications in the Central Nervous System.
Ikezu T, Yang Y, Verderio C, Krämer-Albers EM. Ikezu T, et al. J Neurosci. 2024 Oct 2;44(40):e1170242024. doi: 10.1523/JNEUROSCI.1170-24.2024. J Neurosci. 2024. PMID: 39358029 Review. - Cuproptosis: Mechanisms, biological significance, and advances in disease treatment-A systematic review.
Pan C, Ji Z, Wang Q, Zhang Z, Wang Z, Li C, Lu S, Ge P. Pan C, et al. CNS Neurosci Ther. 2024 Sep;30(9):e70039. doi: 10.1111/cns.70039. CNS Neurosci Ther. 2024. PMID: 39267265 Free PMC article. Review. - Microglia Colonization Associated with Angiogenesis and Neural Cell Development.
Harry GJ. Harry GJ. Adv Neurobiol. 2024;37:163-178. doi: 10.1007/978-3-031-55529-9_10. Adv Neurobiol. 2024. PMID: 39207692 Review. - Mechanisms and Virulence Factors of Cryptococcus neoformans Dissemination to the Central Nervous System.
Al-Huthaifi AM, Radman BA, Al-Alawi AA, Mahmood F, Liu TB. Al-Huthaifi AM, et al. J Fungi (Basel). 2024 Aug 17;10(8):586. doi: 10.3390/jof10080586. J Fungi (Basel). 2024. PMID: 39194911 Free PMC article. Review.
References
- Miller FD, Gauthier AS. Neuron. 2007;54:357. - PubMed
- Hirabayashi Y, et al. Development. 2004;131:2791. - PubMed
- Israsena N, Hu M, Fu W, Kan L, Kessler JA. Dev. Biol. 2004;268:220. - PubMed
- Zhou CJ, Borello U, Rubenstein JL, Pleasure SJ. Neuroscience. 2006;142:1119. - PubMed
- Hirabayashi Y, et al. Neuron. 2009;63:600. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS053538/NS/NINDS NIH HHS/United States
- R37 NS053538/NS/NINDS NIH HHS/United States
- NS053538/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources