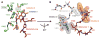Alleviating cancer drug toxicity by inhibiting a bacterial enzyme - PubMed (original) (raw)
Alleviating cancer drug toxicity by inhibiting a bacterial enzyme
Bret D Wallace et al. Science. 2010.
Abstract
The dose-limiting side effect of the common colon cancer chemotherapeutic CPT-11 is severe diarrhea caused by symbiotic bacterial β-glucuronidases that reactivate the drug in the gut. We sought to target these enzymes without killing the commensal bacteria essential for human health. Potent bacterial β-glucuronidase inhibitors were identified by high-throughput screening and shown to have no effect on the orthologous mammalian enzyme. Crystal structures established that selectivity was based on a loop unique to bacterial β-glucuronidases. Inhibitors were highly effective against the enzyme target in living aerobic and anaerobic bacteria, but did not kill the bacteria or harm mammalian cells. Finally, oral administration of an inhibitor protected mice from CPT-11-induced toxicity. Thus, drugs may be designed to inhibit undesirable enzyme activities in essential microbial symbiotes to enhance chemotherapeutic efficacy.
Figures
Fig. 1
CPT-11 metabolism and E. coli β-glucuronidase. (A) Intravenously administered CPT-11 is activated by carboxylesterases (CE) to SN-38, an antineoplastic topoisomerase I poison. Liver SN-38 is inactivated via glucuronidation to SN-38G by UDP-glucuronosyltransferase (UGT) enzymes and sent to the intestines. β-Glucuronidases (β-glucs) in the symbiotic GI bacteria remove the glucuronide as a carbon source, and active SN-38 in the intestinal lumen generates dose-limiting diarrhea. (B) Crystal structure of the E. coli β-glucuronidase tetramer at 2.5 Å resolution. (C) Four selective bacterial β-glucuronidase inhibitors identified via high-throughput screening.
Fig. 2
Potent β-glucuronidase inhibitors. (A) Crystal structures of Inhibitors 2 and 3 bound to the active site of E. coli β-glucuronidase. (B) Inhibitors are observed to stack cooperatively between monomers in the E. coli β-glucuronidase tetramer. Amino acid abbreviations: D, Asp; E, Glu; F, Phe; G, Gly; L, Leu; M, Met; R, Arg; S, Ser; Y, Tyr.
Fig. 3
Inhibitor selectivity for bacterial β-glucuronidase. (A) The 360–376 loop forms direct contact with the bound inhibitors in the E. coli β-glucuronidase structure. This loop is missing from the structure of human β-glucuronidase; thus, it is labeled the “bacterial loop.” (B) Elimination of the “bacterial loop” from E. coli β-glucuronidase produces an enzyme insensitive to inhibitor efficacy. (C) β-Glucuronidase inhibition in living E. coli cells grown under both aerobic and anaerobic conditions. (D) β-Glucuronidase inhibition in two obligate anaerobic bacteria. Error bars represent SD; N = 3.
Fig. 4
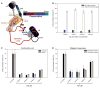
Alleviation of CPT-11 toxicity in mice. (A) CPT-11 produced bloody diarrhea starting after 8 days and peaking at 10 days, whereas oral administration of Inhibitor 1 with CPT-11 reduced the incidence of bloody diarrhea. Vehicle and Inhibitor 1 alone caused no bloody diarrhea. By day 8 to 11, mice in the CPT-11 group began to suffer from severe lethargy and lack of movement; by day 11, all mice in that group were euthanized according to AIC protocol 20070715. (B) Histologic score of the distal and proximal colon of animals in the four treatment groups. Error bars represent SD; N = 12. (C) Tissue histology of colons taken from mice from each treatment group show healthy glandular structure for both vehicle and Inhibitor 1 but highly disrupted tissues in the CPT-11 group. In contrast, Inhibitor 1 provided in combination with CPT-11 protects the colon from damage.
Comment in
- Cancer. Targeting bacteria to improve cancer therapy.
Patel AG, Kaufmann SH. Patel AG, et al. Science. 2010 Nov 5;330(6005):766-7. doi: 10.1126/science.1198310. Science. 2010. PMID: 21051622 No abstract available.
Similar articles
- Cancer. Targeting bacteria to improve cancer therapy.
Patel AG, Kaufmann SH. Patel AG, et al. Science. 2010 Nov 5;330(6005):766-7. doi: 10.1126/science.1198310. Science. 2010. PMID: 21051622 No abstract available. - Molecular insights into microbial β-glucuronidase inhibition to abrogate CPT-11 toxicity.
Roberts AB, Wallace BD, Venkatesh MK, Mani S, Redinbo MR. Roberts AB, et al. Mol Pharmacol. 2013 Aug;84(2):208-17. doi: 10.1124/mol.113.085852. Epub 2013 May 20. Mol Pharmacol. 2013. PMID: 23690068 Free PMC article. - Structure and Inhibition of Microbiome β-Glucuronidases Essential to the Alleviation of Cancer Drug Toxicity.
Wallace BD, Roberts AB, Pollet RM, Ingle JD, Biernat KA, Pellock SJ, Venkatesh MK, Guthrie L, O'Neal SK, Robinson SJ, Dollinger M, Figueroa E, McShane SR, Cohen RD, Jin J, Frye SV, Zamboni WC, Pepe-Ranney C, Mani S, Kelly L, Redinbo MR. Wallace BD, et al. Chem Biol. 2015 Sep 17;22(9):1238-49. doi: 10.1016/j.chembiol.2015.08.005. Epub 2015 Sep 10. Chem Biol. 2015. PMID: 26364932 Free PMC article. - New approaches to prevent intestinal toxicity of irinotecan-based regimens.
Alimonti A, Gelibter A, Pavese I, Satta F, Cognetti F, Ferretti G, Rasio D, Vecchione A, Di Palma M. Alimonti A, et al. Cancer Treat Rev. 2004 Oct;30(6):555-62. doi: 10.1016/j.ctrv.2004.05.002. Cancer Treat Rev. 2004. PMID: 15325035 Review. - Novel agents that potentially inhibit irinotecan-induced diarrhea.
Yang X, Hu Z, Chan SY, Chan E, Goh BC, Duan W, Zhou S. Yang X, et al. Curr Med Chem. 2005;12(11):1343-58. doi: 10.2174/0929867054020972. Curr Med Chem. 2005. PMID: 15975002 Review.
Cited by
- The role of gut microbial β-glucuronidases in carcinogenesis and cancer treatment: a scoping review.
Hillege LE, Stevens MAM, Kristen PAJ, de Vos-Geelen J, Penders J, Redinbo MR, Smidt ML. Hillege LE, et al. J Cancer Res Clin Oncol. 2024 Nov 13;150(11):495. doi: 10.1007/s00432-024-06028-2. J Cancer Res Clin Oncol. 2024. PMID: 39537966 Free PMC article. Review. - Effect of antibiotic drug use on outcome and therapy-related toxicity in patients with glioblastoma-A retrospective cohort study.
Götz L, Ansafi T, Gerken M, Klinkhammer-Schalke M, Fischl A, Riemenschneider MJ, Proescholdt M, Bumes E, Kölbl O, Schmidt NO, Linker R, Hau P, Haedenkamp TM. Götz L, et al. Neurooncol Adv. 2024 Oct 4;6(1):vdae170. doi: 10.1093/noajnl/vdae170. eCollection 2024 Jan-Dec. Neurooncol Adv. 2024. PMID: 39493414 Free PMC article. - EphA2 blockage ALW-II-41-27 alleviates atherosclerosis by remodeling gut microbiota to regulate bile acid metabolism.
Lu C, Liu D, Wu Q, Zeng J, Xiong Y, Luo T. Lu C, et al. NPJ Biofilms Microbiomes. 2024 Oct 19;10(1):108. doi: 10.1038/s41522-024-00585-7. NPJ Biofilms Microbiomes. 2024. PMID: 39426981 Free PMC article. - Three bioactive compounds from Huangqin decoction ameliorate Irinotecan-induced diarrhea via dual-targeting of Escherichia coli and bacterial β-glucuronidase.
Teng X, Wu B, Liang Z, Zhang L, Yang M, Liu Z, Liang Q, Wang C. Teng X, et al. Cell Biol Toxicol. 2024 Oct 18;40(1):88. doi: 10.1007/s10565-024-09922-0. Cell Biol Toxicol. 2024. PMID: 39422738 Free PMC article. - The Role of Microbiome and Probiotics in Chemo-Radiotherapy-Induced Diarrhea: A Narrative Review of the Current Evidence.
Khorashadizadeh S, Abbasifar S, Yousefi M, Fayedeh F, Moodi Ghalibaf A. Khorashadizadeh S, et al. Cancer Rep (Hoboken). 2024 Oct;7(10):e70029. doi: 10.1002/cnr2.70029. Cancer Rep (Hoboken). 2024. PMID: 39410854 Free PMC article. Review.
References
- Hsiang YH, Hertzberg R, Hecht S, Liu LF. J Biol Chem. 1985;260:14873. - PubMed
- Redinbo MR, Champoux JJ, Hol WG. Curr Opin Struct Biol. 1999;9:29. - PubMed
- Pizzolato JF, Saltz LB. Lancet. 2003;361:2235. - PubMed
- Pommier Y. Nat Rev Cancer. 2006;6:789. - PubMed
- Smith NF, Figg WD, Sparreboom A. Toxicol In Vitro. 2006;20:163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA127231-01A2/CA/NCI NIH HHS/United States
- R01 DK073338/DK/NIDDK NIH HHS/United States
- R01 CA127231-02/CA/NCI NIH HHS/United States
- R01 CA161879/CA/NCI NIH HHS/United States
- R01 CA127231-03/CA/NCI NIH HHS/United States
- CA98468/CA/NCI NIH HHS/United States
- R01 CA098468/CA/NCI NIH HHS/United States
- R01 CA127231/CA/NCI NIH HHS/United States
- CA127231/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases