Transforming growth factor (TGF)-β signaling in cardiac remodeling - PubMed (original) (raw)
Review
Transforming growth factor (TGF)-β signaling in cardiac remodeling
Marcin Dobaczewski et al. J Mol Cell Cardiol. 2011 Oct.
Abstract
Myocardial TGF-β expression is upregulated in experimental models of myocardial infarction and cardiac hypertrophy, and in patients with dilated or hypertrophic cardiomyopathy. Through its effects on cardiomyocytes, mesenchymal and immune cells, TGF-β plays an important role in the pathogenesis of cardiac remodeling and fibrosis. TGF-β overexpression in the mouse heart is associated with fibrosis and hypertrophy. Endogenous TGF-β plays an important role in the pathogenesis of cardiac fibrotic and hypertrophic remodeling, and modulates matrix metabolism in the pressure-overloaded heart. In the infarcted heart, TGF-β deactivates inflammatory macrophages, while promoting myofibroblast transdifferentiation and matrix synthesis through Smad3-dependent pathways. Thus, TGF-β may serve as the "master switchThis article is part of a special issue entitled "Key Signaling Molecules in Hypertrophy and Heart Failure". for the transition of the infarct from the inflammatory phase to formation of the scar. Because of its crucial role in cardiac remodeling, the TGF-β system may be a promising therapeutic target for patients with heart failure. However, efforts to translate these concepts into therapeutic strategies, in order to prevent cardiac hypertrophy and fibrosis, are hampered by the complex, pleiotropic and diverse effects of TGF-β signaling, by concerns regarding deleterious actions of TGF-β inhibition and by the possibility of limited benefit in patients receiving optimal treatment with ACE inhibitors and β-adrenergic blockers. Dissection of the pathways responsible for specific TGF-β-mediated actions and understanding of cell-specific actions of TGF-β are needed to design optimal therapeutic strategies. This article is part of a special issue entitled "Key Signaling Molecules in Hypertrophy and Heart Failure".
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
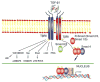
Figure 1
TGF-β signaling pathways. TGF-β transduces its signal through Smad-dependent and Smad-independent pathways.
Figure 2
The cellular effects of TGF-β. TGF-β exerts pleiotropic effects on all cell types involved in cardiac injury, repair and remodeling.
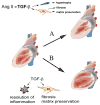
Figure 3
The role of TGF-β in hypertrophy, fibrosis and post-infarction cardiac remodeling. A: TGF-β transduces hypertrophic signals in cardiomyocytes and induces myofibroblast transdifferentiation, while promoting matrix deposition and preservation. Although TGF-β inhibition in the pressure-overloaded ventricle may attenuate hypertrophy and reduce fibrosis protecting from diastolic dysfunction, complete loss of TGF-β signaling may result in unopposed matrix degradation, cardiac dilation and systolic dysfunction. B: In the infarcted heart TGF-β may serve as the “master switch” that regulates transition from the inflammatory phase to scar formation. TGF-β suppresses inflammatory mediator synthesis by macrophages while enhancing myofibroblast transdifferentiation and matrix deposition. Thus, timing is a crucial determinant of outcome in pharmacologic interventions targeting the TGF-β system following myocardial infarction.
Similar articles
- MicroRNA-221/222 Family Counteracts Myocardial Fibrosis in Pressure Overload-Induced Heart Failure.
Verjans R, Peters T, Beaumont FJ, van Leeuwen R, van Herwaarden T, Verhesen W, Munts C, Bijnen M, Henkens M, Diez J, de Windt LJ, van Nieuwenhoven FA, van Bilsen M, Goumans MJ, Heymans S, González A, Schroen B. Verjans R, et al. Hypertension. 2018 Feb;71(2):280-288. doi: 10.1161/HYPERTENSIONAHA.117.10094. Epub 2017 Dec 18. Hypertension. 2018. PMID: 29255073 - Endothelial Forkhead Box Transcription Factor P1 Regulates Pathological Cardiac Remodeling Through Transforming Growth Factor-β1-Endothelin-1 Signal Pathway.
Liu J, Zhuang T, Pi J, Chen X, Zhang Q, Li Y, Wang H, Shen Y, Tomlinson B, Chan P, Yu Z, Cheng Y, Zheng X, Reilly M, Morrisey E, Zhang L, Liu Z, Zhang Y. Liu J, et al. Circulation. 2019 Aug 20;140(8):665-680. doi: 10.1161/CIRCULATIONAHA.119.039767. Epub 2019 Jun 10. Circulation. 2019. PMID: 31177814 - ADAMTS16 activates latent TGF-β, accentuating fibrosis and dysfunction of the pressure-overloaded heart.
Yao Y, Hu C, Song Q, Li Y, Da X, Yu Y, Li H, Clark IM, Chen Q, Wang QK. Yao Y, et al. Cardiovasc Res. 2020 Apr 1;116(5):956-969. doi: 10.1093/cvr/cvz187. Cardiovasc Res. 2020. PMID: 31297506 Free PMC article. - The role of TGF-beta signaling in myocardial infarction and cardiac remodeling.
Bujak M, Frangogiannis NG. Bujak M, et al. Cardiovasc Res. 2007 May 1;74(2):184-95. doi: 10.1016/j.cardiores.2006.10.002. Epub 2006 Oct 7. Cardiovasc Res. 2007. PMID: 17109837 Free PMC article. Review. - TGF-β as a therapeutic target in the infarcted and failing heart: cellular mechanisms, challenges, and opportunities.
Frangogiannis NG. Frangogiannis NG. Expert Opin Ther Targets. 2024 Jan-Feb;28(1-2):45-56. doi: 10.1080/14728222.2024.2316735. Epub 2024 Feb 19. Expert Opin Ther Targets. 2024. PMID: 38329809 Review.
Cited by
- TongGuanWan Alleviates Doxorubicin- and Isoproterenol-Induced Cardiac Hypertrophy and Fibrosis by Modulating Apoptotic and Fibrotic Pathways.
Yoon JJ, Tai AL, Kim HY, Han BH, Shin S, Lee HS, Kang DG. Yoon JJ, et al. Int J Mol Sci. 2024 Sep 30;25(19):10573. doi: 10.3390/ijms251910573. Int J Mol Sci. 2024. PMID: 39408900 Free PMC article. - Pathophysiology of dilated cardiomyopathy: from mechanisms to precision medicine.
Gigli M, Stolfo D, Merlo M, Sinagra G, Taylor MRG, Mestroni L. Gigli M, et al. Nat Rev Cardiol. 2024 Oct 11. doi: 10.1038/s41569-024-01074-2. Online ahead of print. Nat Rev Cardiol. 2024. PMID: 39394525 Review. - Vitamin D-Parathyroid Hormone-Fibroblast Growth Factor 23 Axis and Cardiac Remodeling.
Deng C, Wu Y. Deng C, et al. Am J Cardiovasc Drugs. 2024 Oct 11. doi: 10.1007/s40256-024-00688-8. Online ahead of print. Am J Cardiovasc Drugs. 2024. PMID: 39392562 Review. - Potential diagnostic value of circulating miRNAs in HFrEF and bioinformatics analysis.
Kuai Z, Ma Y, Gao W, Zhang X, Wang X, Ye Y, Zhang X, Yuan J. Kuai Z, et al. Heliyon. 2024 Sep 20;10(19):e37929. doi: 10.1016/j.heliyon.2024.e37929. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39386873 Free PMC article. - Quercetin regulates pulmonary vascular remodeling in pulmonary hypertension by downregulating TGF-β1-Smad2/3 pathway.
Gao RJ, Aikeremu N, Cao N, Chen C, Ma KT, Li L, Zhang AM, Si JQ. Gao RJ, et al. BMC Cardiovasc Disord. 2024 Oct 4;24(1):535. doi: 10.1186/s12872-024-04192-4. BMC Cardiovasc Disord. 2024. PMID: 39367342 Free PMC article.
References
- Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35:83–92. - PubMed
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–24. - PubMed
- Koli K, Saharinen J, Hyytiainen M, Penttinen C, Keski-Oja J. Latency, activation, and binding proteins of TGF-beta. Microsc Res Tech. 2001;52:354–62. - PubMed
- Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL76246/HL/NHLBI NIH HHS/United States
- R01 HL085440/HL/NHLBI NIH HHS/United States
- R01 HL076246-06/HL/NHLBI NIH HHS/United States
- R01 HL076246/HL/NHLBI NIH HHS/United States
- R01 HL085440-03/HL/NHLBI NIH HHS/United States
- R01 HL85440/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous