Functions of T cells in asthma: more than just T(H)2 cells - PubMed (original) (raw)
Review
. 2010 Dec;10(12):838-48.
doi: 10.1038/nri2870. Epub 2010 Nov 9.
Affiliations
- PMID: 21060320
- PMCID: PMC3807783
- DOI: 10.1038/nri2870
Review
Functions of T cells in asthma: more than just T(H)2 cells
Clare M Lloyd et al. Nat Rev Immunol. 2010 Dec.
Abstract
Asthma has been considered a T helper 2 (T(H)2) cell-associated inflammatory disease, and T(H)2-type cytokines, such as interleukin-4 (IL-4), IL-5 and IL-13, are thought to drive the disease pathology in patients. Although atopic asthma has a substantial T(H)2 cell component, the disease is notoriously heterogeneous, and recent evidence has suggested that other T cells also contribute to the development of asthma. Here, we discuss the roles of different T cell subsets in the allergic lung, consider how each subset can contribute to the development of allergic pathology and evaluate how we might manipulate these cells for new asthma therapies.
Trial registration: ClinicalTrials.gov NCT00707811 NCT00712205 NCT00968669.
Figures
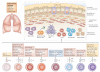
Figure 1. T cells involved in the induction of the allergic phenotype
Asthma is a heterogeneous disease that is characterized by airway hyperresponsiveness (AHR), recruitment of inflammatory leukocytes to the lung and tissue remodelling, including mucus production and airway smooth muscle changes. A number of different T cell subsets are thought to influence the nature and magnitude of the allergic immune response by the cytokines that they secrete. T helper 2 (TH2) cells are thought to promote eosinophil recruitment, in conjunction with nature killer T (NKT) cells and CD8+ T cells. By contrast, TH1 cells and TH17 cells are thought to be associated with severe, steroid-resistant asthma, which is often marked by neutrophilic infiltrates. Regulatory T (TReg) cells and subtypes of γδ T cells are able to downregulate pulmonary immune responses and are thought to be important for maintenance of immune homeostasis in the lungs. The nature and magnitude of allergic inflammation in the lung is influenced by external environmental stimuli, such as exposure to allergens and pollution as well as infection with pathogens. IFNγ, interferon-γ; IL, interleukin.
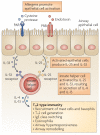
Figure 2. Alternative pathway to a TH2-type response in the airways
Cysteine protease activity and endotoxin within allergens can activate lung epithelial cells through protease-activated receptors (PARs) and pattern-recognition receptors (PRRs), such as Toll-like receptors. Recent experimental data indicate that a population of ‘innate helper’ cells can secrete interleukin-4 (IL-4) and IL-13 in response to epithelial cell-derived cytokines, such as IL-33 and IL-25, and promote T helper 2 (TH2)-type immune responses. Thymic stromal lymphopoietin (TSLP) can promote TH2-type responses in the lung, but a direct association with this cytokine and innate helper cells in the lung has not yet been found. Although these innate helper cells have been identified in a number of different tissues, including in the resting lungs, evidence for their involvement in allergic airway inflammation remains indirect. Therefore the model described here is theoretical and remains to be tested in vivo.
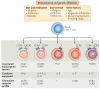
Figure 3. T cell subset signatures are affected by a variety of genetic and environmental influences
T cell subsets can be distinguished by their cytokine secretion patterns, as well as by their chemokine receptor expression. Distinct T cell lineages are determined by the expression of ‘master’ transcription factors. Although once thought to be committed stable lineages, it is becoming apparent that a range of environmental and genetic influences are able to promote flexibility in T cell programmes. Thus, effector subsets can rapidly react to changing environmental circumstances to promote effective immune responses. CCR, CC-chemokine receptor; CXCR, CXC-chemokine receptor; FOXP3, forkhead box P3; GATA3, GATA-binding protein 3; IFNγ, interferon-γ; IL, interleukin; RORγt, retinoic acid receptor-related orphan receptor-γt; STAT, signal transducer and activator of transcription; TH, T helper; TReg, regulatory T.
Similar articles
- Blocking KV1.3 channels inhibits Th2 lymphocyte function and treats a rat model of asthma.
Koshy S, Huq R, Tanner MR, Atik MA, Porter PC, Khan FS, Pennington MW, Hanania NA, Corry DB, Beeton C. Koshy S, et al. J Biol Chem. 2014 May 2;289(18):12623-32. doi: 10.1074/jbc.M113.517037. Epub 2014 Mar 18. J Biol Chem. 2014. PMID: 24644290 Free PMC article. - Evidence for Asthma in the Lungs of Mice Inoculated with Different Doses of Toxocara canis.
Hanh NTL, Lee YL, Lin CL, Chou CM, Cheng PC, Quang HH, Fan CK. Hanh NTL, et al. Am J Trop Med Hyg. 2020 Dec;103(6):2305-2314. doi: 10.4269/ajtmh.20-0484. Epub 2020 Sep 17. Am J Trop Med Hyg. 2020. PMID: 32975177 Free PMC article. - IL-4 increases type 2, but not type 1, cytokine production in CD8+ T cells from mild atopic asthmatics.
Stanciu LA, Roberts K, Papadopoulos NG, Cho SH, Holgate ST, Coyle AJ, Johnston SL. Stanciu LA, et al. Respir Res. 2005 Jul 7;6(1):67. doi: 10.1186/1465-9921-6-67. Respir Res. 2005. PMID: 16001979 Free PMC article. Clinical Trial. - TH2 and 'TH2-like' cells in allergy and asthma: pharmacological perspectives.
Anderson GP, Coyle AJ. Anderson GP, et al. Trends Pharmacol Sci. 1994 Sep;15(9):324-32. doi: 10.1016/0165-6147(94)90027-2. Trends Pharmacol Sci. 1994. PMID: 7992386 Review. - The role of Th2 type CD4+ T cells and Th2 type CD8+ T cells in asthma.
Erb KJ, Le Gros G. Erb KJ, et al. Immunol Cell Biol. 1996 Apr;74(2):206-8. doi: 10.1038/icb.1996.29. Immunol Cell Biol. 1996. PMID: 8724011 Review.
Cited by
- Intestinal mycobiota in health and diseases: from a disrupted equilibrium to clinical opportunities.
Wu X, Xia Y, He F, Zhu C, Ren W. Wu X, et al. Microbiome. 2021 Mar 14;9(1):60. doi: 10.1186/s40168-021-01024-x. Microbiome. 2021. PMID: 33715629 Free PMC article. Review. - The role of Sema4A in angiogenesis, immune responses, carcinogenesis, and retinal systems.
Ito D, Kumanogoh A. Ito D, et al. Cell Adh Migr. 2016 Nov;10(6):692-699. doi: 10.1080/19336918.2016.1215785. Epub 2016 Oct 13. Cell Adh Migr. 2016. PMID: 27736304 Free PMC article. Review. - Human schistosome infection and allergic sensitisation.
Rujeni N, Taylor DW, Mutapi F. Rujeni N, et al. J Parasitol Res. 2012;2012:154743. doi: 10.1155/2012/154743. Epub 2012 Aug 27. J Parasitol Res. 2012. PMID: 22970345 Free PMC article. - The impact of maternal obesity during pregnancy on offspring immunity.
Wilson RM, Messaoudi I. Wilson RM, et al. Mol Cell Endocrinol. 2015 Dec 15;418 Pt 2(0 2):134-42. doi: 10.1016/j.mce.2015.07.028. Epub 2015 Jul 30. Mol Cell Endocrinol. 2015. PMID: 26232506 Free PMC article. Review. - An inhibitory role for Sema4A in antigen-specific allergic asthma.
Morihana T, Goya S, Mizui M, Yasui T, Prasad DV, Kumanogoh A, Tamura M, Shikina T, Maeda Y, Iwamoto Y, Inohara H, Kikutani H. Morihana T, et al. J Clin Immunol. 2013 Jan;33(1):200-9. doi: 10.1007/s10875-012-9798-5. Epub 2012 Sep 25. J Clin Immunol. 2013. PMID: 23007237
References
- Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv. Immunol. 1989;46:111–147. - PubMed
- Robinson DS, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. - PubMed
- Bentley AM, et al. Identification of T lymphocytes, macrophages, and activated eosinophils in the bronchial mucosa in intrinsic asthma. Relationship to symptoms and bronchial responsiveness. Am. Rev. Respir. Dis. 1992;146:500–506. - PubMed
- Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu. Rev. Immunol. 2004;22:789–815. - PubMed
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255–281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical