Neutrophil extracellular trap cell death requires both autophagy and superoxide generation - PubMed (original) (raw)
Neutrophil extracellular trap cell death requires both autophagy and superoxide generation
Quinten Remijsen et al. Cell Res. 2011 Feb.
Abstract
Neutrophil extracellular traps (NETs) are extracellular chromatin structures that can trap and degrade microbes. They arise from neutrophils that have activated a cell death program called NET cell death, or NETosis. Activation of NETosis has been shown to involve NADPH oxidase activity, disintegration of the nuclear envelope and most granule membranes, decondensation of nuclear chromatin and formation of NETs. We report that in phorbol myristate acetate (PMA)-stimulated neutrophils, intracellular chromatin decondensation and NET formation follow autophagy and superoxide production, both of which are required to mediate PMA-induced NETosis and occur independently of each other. Neutrophils from patients with chronic granulomatous disease, which lack NADPH oxidase activity, still exhibit PMA-induced autophagy. Conversely, PMA-induced NADPH oxidase activity is not affected by pharmacological inhibition of autophagy. Interestingly, inhibition of either autophagy or NADPH oxidase prevents intracellular chromatin decondensation, which is essential for NETosis and NET formation, and results in cell death characterized by hallmarks of apoptosis. These results indicate that apoptosis might function as a backup program for NETosis when autophagy or NADPH oxidase activity is prevented.
Figures
Figure 1
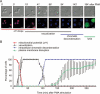
PMA-induced NETosis is characterized, chronologically, by cell flattening and adherence, a drop in mitochondrial membrane potential, vacuolization and intracellular chromatin decondensation. (A) Isolated neutrophils (15 × 104 PMN per ml) were monitored by live cell imaging for four parameters: morphology using differential interface contrast (DIC), mitochondrial potential using TMRM (red), cell death using the cell-impermeable DNA dye Sytox Green (green) and chromatin decondensation using the cell-permeable DNA marker Hoechst 33342 (blue). These were performed in a humidified atmosphere containing 5% CO2 at 37 °C. Cells were stimulated with 100 nM PMA and monitored every min for up to 300 min. Important time points are shown in min. Vacuoles are indicated by arrows, and scale bars represent 10 μm. Results are representative of at least four independent experiments. (B) Kinetic analysis of 150 cells from four independent experiments. Shown is the mean ± SD of the percentage of cells undergoing subcellular events associated with cell death (loss of mitochondrial potential, vacuolization, intracellular chromatin decondensation and plasma membrane permeabilization).
Figure 2
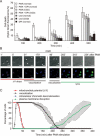
Absence of Nox2 activity blocks PMA-induced intracellular chromatin decondensation and delays cell death, whereas vacuolization and the drop in mitochondrial membrane potential are unaffected. (A) Normal and CGD neutrophils (2 × 105) were incubated with or without 10 μM DPI for 30 min, and either left unstimulated or were stimulated with 100 nM PMA for the indicated periods in the presence of the cell-impermeable DNA dye Sytox Green (50 nM). Data are expressed as percentage of maximal Sytox Green fluorescence ± SD (n = 3) as a function of time. *P < 0.01 and **P < 0.001, as compared to PMA-stimulated normal neutrophils. (B) Isolated neutrophils obtained from a CGD patient were examined by live cell imaging for different parameters in a humidified atmosphere containing 5% CO2 at 37 °C. Left panel: morphology using DIC, mitochondrial potential using TMRM (red), and chromatin decondensation using the cell-permeable DNA marker Hoechst 33342 (blue). Right panel: morphology using DIC, chromatin decondensation using the cell-permeable DNA marker Hoechst 33342 (blue) and cell death using the cell-impermeable DNA dye PI (green). Cells were stimulated with 100 nM PMA and monitored every min for up to 400 min. Important time points are shown in min. Vacuoles are indicated by arrows, and scale bars represent 10 μm. (C) Kinetic analysis of 85 cells from three independent experiments. Shown is the mean ± SD of the percentage of cells undergoing subcellular events associated with cell death (loss of mitochondrial potential, vacuolization, intracellular chromatin decondensation and plasma membrane permeabilization).
Figure 3
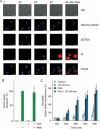
PMA-induced vacuolization is preceded by typical features of autophagy. (A) Human neutrophils (3 × 105 PMN per ml) were left unstimulated or were stimulated with 100 nM PMA for 15, 30, 80 or 120 min. Cells were then fixed and examined by transmission electron microscopy (TEM). (B) Zoom in on TEM picture of human neutrophils stimulated with 100 nM PMA for 30 min. De novo formation of isolation membranes (i) (1). Engulfment of cytoplasmic content (2), such as granules or ribosomes (arrows). Generation of early immature autophagic vacuoles (Avi) (3) characterized by a double phospholipid bilayer (arrowheads). Degradation of vacuolar content on fusion with endosomes/lysosomes in late degradative autophagic vacuole (Avd) (4). (C) PMA stimulation induces recruitment of LC3 to autophagosomes. Normal neutrophils and neutrophils from CGD patients were left unstimulated or were stimulated for 15 min with 100 nM PMA and then fixed, permeabilized and stained for LC3 and for nuclear chromatin, and analyzed by confocal microscopy. DIC and fluorescent images are shown. Scale bars represent 10 μm. Representative results of three independent experiments are shown. In (A and B) the scale bars for full cell images indicate 1 μm and for magnified cell areas 100 nm. Nuclear lobi are indicated (N).
Figure 4
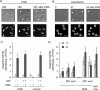
Inhibition of autophagy by wortmannin blocks vacuolization and intracellular chromatin decondensation, but generation of ROS is unaffected. (A) Human neutrophils were incubated with the ROS probe DCFDA (green) and the cell-permeable DNA dye Hoechst 33342 (blue) for 30 min at 37 °C in a humidified atmosphere containing 5% CO2. Subsequently, medium containing the cell-impermeable DNA marker PI (red) and wortmannin (100 nM) was refreshed. Cells were stimulated with 100 nM PMA and monitored every min by live cell imaging. Important time points are shown in min. Representative results of three independent experiments are shown. The scale bars represent 10 μm. (B) Cells were either untreated or pretreated with wortmannin (100 nM) for 30 min, and then stimulated with 100 nM PMA. Superoxide production was determined by enhanced chemiluminescence. Mean maximal superoxide production is expressed as percentage of control ± SD (n = 3). (C) In the presence of 50 nM Sytox Green, 2 × 105 neutrophils were incubated with or without wortmannin (100 nM) for 30 min and were either left unstimulated or stimulated with 100 nM PMA for the indicated periods. Cell death was detected by measuring the fluorescence of the cell-impermeable DNA dye Sytox Green. Data are expressed as percentage of maximal Sytox Green fluorescence ± SD (n = 3) as a function of time. *P < 0.05 and **P < 0.01, as compared to PMA-stimulated normal neutrophils.
Figure 5
PMA induces features of apoptosis when Nox2 or autophagy is inhibited. (A) Live cell images of neutrophils isolated from a CGD patient and stimulated with 100 nM PMA. Morphology was examined using DIC and chromatin decondensation using the cell-permeable DNA marker Hoechst 33342 (blue). Cells were monitored every min for up to 300 min, and important time points are shown in min. PMA stimulation of CGD neutrophils induced membrane blebbing (arrows). (B) Live cell images of isolated neutrophils pretreated with wortmannin (100 nM) for 30 min. Morphology was examined using DIC and chromatin decondensation using the cell-permeable DNA marker Hoechst 33342 (blue). PMA-induced membrane blebbing (arrows) in normal neutrophils pretreated with wortmannin. (C) Normal and CGD neutrophils were incubated with or without DPI (10 μM) for 30 min. Samples were collected (white bars) and the remainder was further stimulated with 100 nM PMA for 3 h (gray bars). Protease activity against the caspase substrate DEVD-amc was determined. Data are expressed as the mean ΔF/min ± SD (n = 3). (D) Normal neutrophils were incubated for 30 min with 10 μM DPI or 100 nM wortmannin (wort) or left untreated. Subsequently, cells were further stimulated for 1 h (light gray bars) or 3 h (dark gray bars) with PMA (100 nM) or with agonistic anti-Fas antibody (250 ng/ml) and analyzed for DEVDase activity. Data are expressed as the mean ΔF/min ± SD (n = 3). *P < 0.01 and **P < 0.001, as compared to PMA-stimulated neutrophils.
Figure 6
(A) PMA-induced NETosis requires both autophagy and superoxide production, which trigger the intracellular chromatin decondensation preceding NET formation. (B) Pharmacological (DPI) or genetic (CGD) inactivation of Nox2 activity prevented PMA-induced superoxide production, but not PMA-induced autophagy. No intracellular chromatin decondensation occurred, and despite massive vacuolization, cells finally underwent cell death characterized by features of apoptosis. (C) Pharmacological (wortmannin) inhibition of PMA-induced autophagy did not interfere with PMA-induced superoxide production. Nevertheless, intracellular chromatin decondensation did not occur, and cells underwent cell death characterized by features of apoptosis.
Similar articles
- Histone Acetylation Promotes Neutrophil Extracellular Trap Formation.
Hamam HJ, Khan MA, Palaniyar N. Hamam HJ, et al. Biomolecules. 2019 Jan 18;9(1):32. doi: 10.3390/biom9010032. Biomolecules. 2019. PMID: 30669408 Free PMC article. - Hypertonic Saline Suppresses NADPH Oxidase-Dependent Neutrophil Extracellular Trap Formation and Promotes Apoptosis.
Nadesalingam A, Chen JHK, Farahvash A, Khan MA. Nadesalingam A, et al. Front Immunol. 2018 Mar 8;9:359. doi: 10.3389/fimmu.2018.00359. eCollection 2018. Front Immunol. 2018. PMID: 29593709 Free PMC article. - Respiratory burst of rabbit peritoneal neutrophils. Transition from an NADPH diaphorase activity to an .O2(-)-generating oxidase activity.
Laporte F, Doussiere J, Vignais PV. Laporte F, et al. Eur J Biochem. 1990 Nov 26;194(1):301-8. doi: 10.1111/j.1432-1033.1990.tb19457.x. Eur J Biochem. 1990. PMID: 2174779 - How Do ROS Induce NETosis? Oxidative DNA Damage, DNA Repair, and Chromatin Decondensation.
Azzouz D, Palaniyar N. Azzouz D, et al. Biomolecules. 2024 Oct 16;14(10):1307. doi: 10.3390/biom14101307. Biomolecules. 2024. PMID: 39456240 Free PMC article. Review. - The roles of NADPH oxidase in modulating neutrophil effector responses.
Zeng MY, Miralda I, Armstrong CL, Uriarte SM, Bagaitkar J. Zeng MY, et al. Mol Oral Microbiol. 2019 Apr;34(2):27-38. doi: 10.1111/omi.12252. Epub 2019 Feb 7. Mol Oral Microbiol. 2019. PMID: 30632295 Free PMC article. Review.
Cited by
- Escaping Underground Nets: Extracellular DNases Degrade Plant Extracellular Traps and Contribute to Virulence of the Plant Pathogenic Bacterium Ralstonia solanacearum.
Tran TM, MacIntyre A, Hawes M, Allen C. Tran TM, et al. PLoS Pathog. 2016 Jun 23;12(6):e1005686. doi: 10.1371/journal.ppat.1005686. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27336156 Free PMC article. - Neutrophil extracellular trap formation is associated with autophagy-related signalling in ANCA-associated vasculitis.
Tang S, Zhang Y, Yin SW, Gao XJ, Shi WW, Wang Y, Huang X, Wang L, Zou LY, Zhao JH, Huang YJ, Shan LY, Gounni AS, Wu YZ, Zhang JB. Tang S, et al. Clin Exp Immunol. 2015 Jun;180(3):408-18. doi: 10.1111/cei.12589. Epub 2015 Apr 29. Clin Exp Immunol. 2015. PMID: 25644394 Free PMC article. - Therapeutic Potential and Immunomodulatory Role of Coenzyme Q10 and Its Analogues in Systemic Autoimmune Diseases.
López-Pedrera C, Villalba JM, Patiño-Trives AM, Luque-Tévar M, Barbarroja N, Aguirre MÁ, Escudero-Contreras A, Pérez-Sánchez C. López-Pedrera C, et al. Antioxidants (Basel). 2021 Apr 13;10(4):600. doi: 10.3390/antiox10040600. Antioxidants (Basel). 2021. PMID: 33924642 Free PMC article. Review. - Characterization of neutrophils and macrophages from ex vivo-cultured murine bone marrow for morphologic maturation and functional responses by imaging flow cytometry.
Pelletier MG, Szymczak K, Barbeau AM, Prata GN, O'Fallon KS, Gaines P. Pelletier MG, et al. Methods. 2017 Jan 1;112:124-146. doi: 10.1016/j.ymeth.2016.09.005. Epub 2016 Sep 20. Methods. 2017. PMID: 27663441 Free PMC article. - The Small GTPase Cdc42 Negatively Regulates the Formation of Neutrophil Extracellular Traps by Engaging Mitochondria.
Tackenberg H, Möller S, Filippi MD, Laskay T. Tackenberg H, et al. Front Immunol. 2021 Feb 17;12:564720. doi: 10.3389/fimmu.2021.564720. eCollection 2021. Front Immunol. 2021. PMID: 33679729 Free PMC article.
References
- Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol. 2008;83:288–295. - PubMed
- Maianski NA, Maianski AN, Kuijpers TW, Roos D. Apoptosis of neutrophils. Acta Haematol. 2004;111:56–66. - PubMed
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. - PubMed
- Steinberg BE, Grinstein S. Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci STKE. 2007;2007:e11. - PubMed
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous