Safety and immunogenicity following administration of a live, attenuated monovalent 2009 H1N1 influenza vaccine to children and adults in two randomized controlled trials - PubMed (original) (raw)
Randomized Controlled Trial
Safety and immunogenicity following administration of a live, attenuated monovalent 2009 H1N1 influenza vaccine to children and adults in two randomized controlled trials
Raburn M Mallory et al. PLoS One. 2010.
Abstract
Background: The safety, tolerability, and immunogenicity of a monovalent intranasal 2009 A/H1N1 live attenuated influenza vaccine (LAIV) were evaluated in children and adults.
Methods/principal findings: Two randomized, double-blind, placebo-controlled studies were completed in children (2-17 y) and adults (18-49 y). Subjects were assigned 4:1 to receive 2 doses of H1N1 LAIV or placebo 28 days apart. The primary safety endpoint was fever ≥38.3°C during days 1-8 after the first dose; the primary immunogenicity endpoint was the proportion of subjects experiencing a postdose seroresponse. Solicited symptoms and adverse events were recorded for 14 days after each dose and safety data were collected for 180 days post-final dose. In total, 326 children (H1N1 LAIV, n = 261; placebo, n = 65) and 300 adults (H1N1 LAIV, n = 240; placebo, n = 60) were enrolled. After dose 1, fever ≥38.3°C occurred in 4 (1.5%) pediatric vaccine recipients and 1 (1.5%) placebo recipient (rate difference, 0%; 95% CI: -6.4%, 3.1%). No adults experienced fever following dose 1. Seroresponse rates in children (H1N1 LAIV vs. placebo) were 11.1% vs. 6.3% after dose 1 (rate difference, 4.8%; 95% CI: -9.6%, 13.8%) and 32.0% vs. 14.5% after dose 2 (rate difference, 17.5%; 95% CI: 5.5%, 27.1%). Seroresponse rates in adults were 6.1% vs. 0% (rate difference, 6.1%; 95% CI: -5.6%, 12.6%) and 14.9% vs. 5.6% (rate difference, 9.3%; 95% CI: -0.8%, 16.3%) after dose 1 and dose 2, respectively. Solicited symptoms after dose 1 (H1N1 LAIV vs. placebo) occurred in 37.5% vs. 32.3% of children and 41.7% vs. 31.7% of adults. Solicited symptoms occurred less frequently after dose 2 in adults and children. No vaccine-related serious adverse events occurred.
Conclusions/significance: In subjects aged 2 to 49 years, two doses of H1N1 LAIV have a safety and immunogenicity profile similar to other previously studied and efficacious formulations of seasonal trivalent LAIV.
Trial registration: ClinicalTrials.gov NCT00946101, NCT00945893.
Conflict of interest statement
Competing Interests: All authors have the following competing interests: 1) Financial: Paid employment by MedImmune, 2) Own stock or shares in MedImmune/AstraZeneca. This affiliation does not affect the authors' adherence to all PLoS ONE policies on sharing data and materials.
Figures
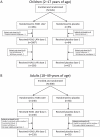
Figure 1. Subject Disposition (ITT Population): (A) Children and (B) Adults.
ITT = intent to treat; LAIV = live attenuated influenza vaccine. *One child randomized to receive H1N1 LAIV was inadvertently administered placebo for dose 1; this subject also received placebo for dose 2. This subject was included in the H1N1 group for ITT analyses, but was grouped with placebo subjects for safety analyses.
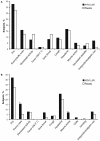
Figure 2. Solicited Symptoms in (A) Children and (B) Adults Through Day 8 Postvaccination with Dose 1.
*P<0.05.
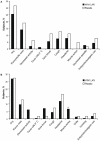
Figure 3. Solicited Symptoms in (A) Children and (B) Adults Through Day 8 Postvaccination with Dose 2.
*P<0.05.
Similar articles
- A study to evaluate the immunogenicity and shedding of live attenuated influenza vaccine strains in children 24-<48 months of age.
Mallory RM, Nyborg A, Kalyani RN, Yuan Y, Block SL, Dubovsky F. Mallory RM, et al. Vaccine. 2020 Jan 29;38(5):1001-1008. doi: 10.1016/j.vaccine.2019.11.055. Epub 2019 Nov 30. Vaccine. 2020. PMID: 31796225 Clinical Trial. - Safety and immunogenicity of a live attenuated influenza H5 candidate vaccine strain A/17/turkey/Turkey/05/133 H5N2 and its priming effects for potential pre-pandemic use: a randomised, double-blind, placebo-controlled trial.
Pitisuttithum P, Boonnak K, Chamnanchanunt S, Puthavathana P, Luvira V, Lerdsamran H, Kaewkungwal J, Lawpoolsri S, Thanachartwet V, Silachamroon U, Masamae W, Schuetz A, Wirachwong P, Thirapakpoomanunt S, Rudenko L, Sparrow E, Friede M, Kieny MP. Pitisuttithum P, et al. Lancet Infect Dis. 2017 Aug;17(8):833-842. doi: 10.1016/S1473-3099(17)30240-2. Epub 2017 May 19. Lancet Infect Dis. 2017. PMID: 28533093 Free PMC article. Clinical Trial. - Immunogenicity and Viral Shedding of Russian-Backbone, Seasonal, Trivalent, Live, Attenuated Influenza Vaccine in a Phase II, Randomized, Placebo-Controlled Trial Among Preschool-Aged Children in Urban Bangladesh.
Lewis KDC, Ortiz JR, Rahman MZ, Levine MZ, Rudenko L, Wright PF, Katz JM, Dally L, Rahman M, Isakova-Sivak I, Ilyushina NA, Matyushenko V, Fry AM, Lindstrom SE, Bresee JS, Brooks WA, Neuzil KM. Lewis KDC, et al. Clin Infect Dis. 2019 Aug 16;69(5):777-785. doi: 10.1093/cid/ciy1003. Clin Infect Dis. 2019. PMID: 30481272 Free PMC article. Clinical Trial. - Live attenuated influenza vaccine (FluMist®; Fluenz™): a review of its use in the prevention of seasonal influenza in children and adults.
Carter NJ, Curran MP. Carter NJ, et al. Drugs. 2011 Aug 20;71(12):1591-622. doi: 10.2165/11206860-000000000-00000. Drugs. 2011. PMID: 21861544 Review. - Live attenuated intranasal influenza vaccine.
Esposito S, Montinaro V, Groppali E, Tenconi R, Semino M, Principi N. Esposito S, et al. Hum Vaccin Immunother. 2012 Jan;8(1):76-80. doi: 10.4161/hv.8.1.18809. Epub 2012 Jan 1. Hum Vaccin Immunother. 2012. PMID: 22251995 Review.
Cited by
- Noninvasive vaccination against infectious diseases.
Zheng Z, Diaz-Arévalo D, Guan H, Zeng M. Zheng Z, et al. Hum Vaccin Immunother. 2018 Jul 3;14(7):1717-1733. doi: 10.1080/21645515.2018.1461296. Epub 2018 May 17. Hum Vaccin Immunother. 2018. PMID: 29624470 Free PMC article. Review. - Systems scale interactive exploration reveals quantitative and qualitative differences in response to influenza and pneumococcal vaccines.
Obermoser G, Presnell S, Domico K, Xu H, Wang Y, Anguiano E, Thompson-Snipes L, Ranganathan R, Zeitner B, Bjork A, Anderson D, Speake C, Ruchaud E, Skinner J, Alsina L, Sharma M, Dutartre H, Cepika A, Israelsson E, Nguyen P, Nguyen QA, Harrod AC, Zurawski SM, Pascual V, Ueno H, Nepom GT, Quinn C, Blankenship D, Palucka K, Banchereau J, Chaussabel D. Obermoser G, et al. Immunity. 2013 Apr 18;38(4):831-44. doi: 10.1016/j.immuni.2012.12.008. Immunity. 2013. PMID: 23601689 Free PMC article. - Live Attenuated Reassortant Vaccines Based on A/Leningrad/134/17/57 Master Donor Virus Against H5 Avian Influenza.
Kiseleva I, Larionova N, Rudenko L. Kiseleva I, et al. Open Microbiol J. 2017 Nov 30;11:316-329. doi: 10.2174/1874285801711010316. eCollection 2017. Open Microbiol J. 2017. PMID: 29290844 Free PMC article. Review. - Vaccines for preventing influenza in healthy adults.
Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Demicheli V, et al. Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD001269. doi: 10.1002/14651858.CD001269.pub6. Cochrane Database Syst Rev. 2018. PMID: 29388196 Free PMC article. - Decreased serologic response in vaccinated military recruits during 2011 correspond to genetic drift in concurrent circulating pandemic A/H1N1 viruses.
Faix DJ, Hawksworth AW, Myers CA, Hansen CJ, Ortiguerra RG, Halpin R, Wentworth D, Pacha LA, Schwartz EG, Garcia SM, Eick-Cost AA, Clagett CD, Khurana S, Golding H, Blair PJ. Faix DJ, et al. PLoS One. 2012;7(4):e34581. doi: 10.1371/journal.pone.0034581. Epub 2012 Apr 13. PLoS One. 2012. PMID: 22514639 Free PMC article.
References
- Maassab HF. Biologic and immunologic characteristics of cold-adapted influenza virus. J Immunol. 1969;102:728–732. - PubMed
- Jin H, Lu B, Zhou H, Ma C, Zhao J, et al. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology. 2003;306:18–24. - PubMed
- Belshe R, Lee MS, Walker RE, Stoddard J, Mendelman PM. Safety, immunogenicity and efficacy of intranasal, live attenuated influenza vaccine. Expert Rev Vaccines. 2004;3:643–654. - PubMed
- Englund JA, Walter EB, Fairchok MP, Monto AS, Neuzil KM. A comparison of 2 influenza vaccine schedules in 6- to 23-month-old children. Pediatrics. 2005;115:1039–1047. - PubMed
- Neuzil KM, Jackson LA, Nelson J, Klimov A, Cox N, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5-8-year-old children. J Infect Dis. 2006;194:1032–1039. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical