Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice - PubMed (original) (raw)
Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice
Noah Saederup et al. PLoS One. 2010.
Erratum in
- Correction: Selective Chemokine Receptor Usage by Central Nervous System Myeloid Cells in CCR2-Red Fluorescent Protein Knock-In Mice.
Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF. Saederup N, et al. PLoS One. 2017 Apr 27;12(4):e0176931. doi: 10.1371/journal.pone.0176931. eCollection 2017. PLoS One. 2017. PMID: 28448577 Free PMC article.
Abstract
Background: Monocyte subpopulations distinguished by differential expression of chemokine receptors CCR2 and CX3CR1 are difficult to track in vivo, partly due to lack of CCR2 reagents.
Methodology/principal findings: We created CCR2-red fluorescent protein (RFP) knock-in mice and crossed them with CX3CR1-GFP mice to investigate monocyte subset trafficking. In mice with experimental autoimmune encephalomyelitis, CCR2 was critical for efficient intrathecal accumulation and localization of Ly6C(hi)/CCR2(hi) monocytes. Surprisingly, neutrophils, not Ly6C(lo) monocytes, largely replaced Ly6C(hi) cells in the central nervous system of these mice. CCR2-RFP expression allowed the first unequivocal distinction between infiltrating monocytes/macrophages from resident microglia.
Conclusion/significance: These results refine the concept of monocyte subsets, provide mechanistic insight about monocyte entry into the central nervous system, and present a novel model for imaging and quantifying inflammatory myeloid populations.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
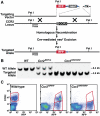
Figure 1. Generation and characterization of CCR2RFP mice.
(A) Recombinant targeting strategy. Hatched box represents region of CCR2 deleted by recombination. Triangles represent loxP sites. (B) Southern blot analysis of WT, Ccr2RFP/+ heterozygous, and Ccr2RFP/RFP homozygous genomic DNA. DNA was digested with _Pst_I, and hybridized with the indicated probe. (C) Flow cytometry analysis of peripheral blood leukocytes (PBL) from WT, Ccr2RFP/+, and Ccr2RFP/RFP mice. Cells were stained with anti-CCR2 antibody MC21. The results are representative of four similar experiments.
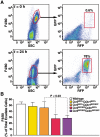
Figure 2. Expression of CCR2 and RFP in sorted cell populations.
(A) CD115+ monocytes were sorted by flow cytometry into populations that did or did not express cell-surface CCR2. Total RNA was isolated, pooled from three mice per genotype, and analyzed for CCR2 mRNA and RFP mRNA by quantitative RT-PCR. Probe specificity was confirmed with WT and Ccr2−/− cells. Expression levels were normalized to beta-actin, and the results are expressed relative to the concentration of CCR2 mRNA in monocytes expressing cell-surface CCR2. (B) NK and T cells were sorted by flow cytometry into RFP+ and RFP– fractions. Expression of CCR2 and RFP mRNA was characterized as in (A) and normalized to the amount of CCR2 RNA in the RFP+ T-cell fraction from Ccr2+/RFP mice. (C to G) WT, Ccr2RFP/+, and Ccr2RFP/RFP mice were bred onto the Apoe−/− background and fed a high-fat diet for 8 weeks. Peripheral blood monocytes were stained for CD115, CCR2, and Ly6C and analyzed by flow cytometry. (C) Gating on monocytes. (D) Ly6C and RFP expression of monocytes. Dashed line indicates the cutoff for a positive RFP signal. (E) Mean RFP fluorescence intensity in Ly6Chi and Ly6Clo subsets. Bars indicate SEM. n = 4 mice/group. P<0.008. (F) CCR2 and RFP expression of Ly6Chi monocytes. (G) CCR2 and RFP expression in Ly6Clo monocytes. CCR2-positive and CCR2-negative gates were based on CCR2-deficient Ccr2RFP/RFP mice. (H to K) Flow cytometric analysis of Ly6C, RFP, GFP and cell-surface CCR2 expression on monocytes from Ccr2RFP/+Cx3cr1GFP/+ mice. CD115+ monocytes were gated (H) by Ly6C/GFP expression and analyzed for RFP (I) and CCR2 (J) expression. Ccr2RFP/RFPCx3cr1GFP/+ monocytes (K) were used as a negative control for CCR2 surface-staining. Results are representative of four mice in two similar experiments.
Figure 3. Analysis of peritoneal exudate cells from Ccr2-RFP Cx3cr1-GFP mice in a sterile peritonitis model.
WT, Ccr2RFP/+Cx3cr1GFP/+, Ccr2RFP/+Cxc3r1GFP/GFP, Ccr2_RFP/RFPCx3cr1GFP/+_, and Ccr2RFP/RFPCx3cr1GFP/GFP mice were injected intraperitoneally with thioglycollate, and inflammatory exudate cells were harvested 24 h later. Cells were stained with F4/80. (A) RFP/GFP profiles of F4/80+ peritoneal cells from naive mice (top panels) and 24 hr after injection (bottom panels). (B) Percentage of exudate cells staining positive for F4/80. Values are mean ± SEM. n = 4 mice/group. Results are representative of two similar experiments.
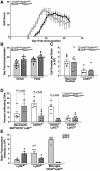
Figure 4. CCR2-deficient mice exhibit delayed onset EAE and decreased monocyte recruitment to the CNS.
(A) Ccr2RFP/+Cx3cr1GFP/+ and Ccr2RFP/RFPCx3cr1GFP/+ mice were immunized with MOG peptide 33–55 and scored daily for neurological signs. (B) EAE symptom onset and peak disease. (C) Total number of brain mononuclear cells and CD45hi infiltrating cells at peak disease. (D) CD45hi infiltrating cells were analyzed to quantify total neutrophils (SSChi, CD115−, Ly6Clo), monocytes (CD115+), and monocyte subsets (CD115+/Ly6Chi/lo), (E) CX3CR1 GFP and CCR2-RFP fluorescence intensities in monocyte subsets and microglia. Data points represent individual mice. Values are mean ± SEM. Results are representative of two similar experiments.
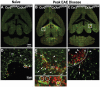
Figure 5. Expression of CX3CR1 and CCR2 in brain lesions of mice with EAE.
(A–C) Low-magnification epifluorescence images of naive Ccr2RFP/+Cx3cr1GFP/+ (A), diseased Ccr2RFP/+Cx3cr1GFP/+ (B), and Ccr2RFP/RFPCx3cr1GFP/+ (C) brains show the extent of inflammation and anatomical location of the lesions. Higher magnification views of the boxed areas are shown in panels D–F. Confocal image of healthy perivascular (D) tissue shows GFP+ microglia (asterisks), small round RFP+ cells (arrowhead), and double-positive cells (circles). (E) Parenchymal lesion in a _Ccr2RFP/+Cx3cr1GFP/+_mouse and (F) a perivascular lesion from a Ccr2RFP/RFPCx3cr1GFP/+ mouse at peak EAE disease. Dashed line in (F) indicates the approximate perivascular boundary. Within EAE lesions, note RFP/GFP double-positive cells (circles), GFP+ activated microglia (E, F; asterisks), large elongated RFP+ cells (E, F; arrows), and small round RFP+ cells (E, F; arrowheads). Small RFP+ cells were CD3+ (not shown). The particularly elongated RFP+ cells in the Ccr2RFP/+Cx3cr1GFP/+ lesion (E) were much less abundant in the Ccr2RFP/RFPCx3cr1GFP/+ lesion (F).
Figure 6. Analysis of monocyte subsets in brain lesions of EAE Ccr2RFP/+Cx3cr1GFP/+ mice at peak EAE.
Confocal images of the periventricular lesions were obtained after staining with the 7/4 antibody. Images represent the same lesion (A) Merged images of red/CCR2, green/CX3CR1, and blue/7/4. (B) Red/CCR2 and green/CX3CR1. (C) Blue/7/4 and green/CX3CR1. (D) Red/CCR2 and blue/7/4. The majority of CCR2+ cells are 7/4+ and CX3CR1lo or negative, suggesting that they are classical infiltrating monocytes (see individual cell “1”). Ly6Clo monocytes (CX3CR1hi and CCR2) are also present (cell “2”), as well as T cells (“3”) and activated microglial cells (“4”).
Similar articles
- Spatio-temporal profile, phenotypic diversity, and fate of recruited monocytes into the post-ischemic brain.
Garcia-Bonilla L, Faraco G, Moore J, Murphy M, Racchumi G, Srinivasan J, Brea D, Iadecola C, Anrather J. Garcia-Bonilla L, et al. J Neuroinflammation. 2016 Nov 4;13(1):285. doi: 10.1186/s12974-016-0750-0. J Neuroinflammation. 2016. PMID: 27814740 Free PMC article. - Expression pattern of Ccr2 and Cx3cr1 in inherited retinal degeneration.
Kohno H, Koso H, Okano K, Sundermeier TR, Saito S, Watanabe S, Tsuneoka H, Sakai T. Kohno H, et al. J Neuroinflammation. 2015 Oct 12;12:188. doi: 10.1186/s12974-015-0408-3. J Neuroinflammation. 2015. PMID: 26458944 Free PMC article. - Gas6 Promotes Inflammatory (CCR2hiCX3CR1lo) Monocyte Recruitment in Venous Thrombosis.
Laurance S, Bertin FR, Ebrahimian T, Kassim Y, Rys RN, Lehoux S, Lemarié CA, Blostein MD. Laurance S, et al. Arterioscler Thromb Vasc Biol. 2017 Jul;37(7):1315-1322. doi: 10.1161/ATVBAHA.116.308925. Epub 2017 Apr 27. Arterioscler Thromb Vasc Biol. 2017. PMID: 28450294 - Monocyte populations are involved in the pathogenesis of experimental epidermolysis bullosa acquisita.
Akbarzadeh R, Czyz C, Thomsen SY, Schilf P, Murthy S, Sadik CD, König P. Akbarzadeh R, et al. Front Immunol. 2023 Dec 5;14:1241461. doi: 10.3389/fimmu.2023.1241461. eCollection 2023. Front Immunol. 2023. PMID: 38116004 Free PMC article. - Role of CCR2 in inflammatory conditions of the central nervous system.
Chu HX, Arumugam TV, Gelderblom M, Magnus T, Drummond GR, Sobey CG. Chu HX, et al. J Cereb Blood Flow Metab. 2014 Sep;34(9):1425-9. doi: 10.1038/jcbfm.2014.120. Epub 2014 Jul 2. J Cereb Blood Flow Metab. 2014. PMID: 24984897 Free PMC article. Review.
Cited by
- Adeno-associated virus delivered CXCL9 sensitizes glioblastoma to anti-PD-1 immune checkpoint blockade.
von Roemeling CA, Patel JA, Carpenter SL, Yegorov O, Yang C, Bhatia A, Doonan BP, Russell R, Trivedi VS, Klippel K, Ryu DH, Grippin A, Futch HS, Ran Y, Hoang-Minh LB, Weidert FL, Golde TE, Mitchell DA. von Roemeling CA, et al. Nat Commun. 2024 Jul 12;15(1):5871. doi: 10.1038/s41467-024-49989-1. Nat Commun. 2024. PMID: 38997283 Free PMC article. - Live cell imaging to understand monocyte, macrophage, and dendritic cell function in atherosclerosis.
McArdle S, Mikulski Z, Ley K. McArdle S, et al. J Exp Med. 2016 Jun 27;213(7):1117-31. doi: 10.1084/jem.20151885. Epub 2016 Jun 6. J Exp Med. 2016. PMID: 27270892 Free PMC article. Review. - Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth.
Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, Rich JN, Bao S. Zhou W, et al. Nat Cell Biol. 2015 Feb;17(2):170-82. doi: 10.1038/ncb3090. Epub 2015 Jan 12. Nat Cell Biol. 2015. PMID: 25580734 Free PMC article. - Bone Fracture Pre-Ischemic Stroke Exacerbates Ischemic Cerebral Injury in Mice.
Wang L, Kang S, Zou D, Zhan L, Li Z, Zhu W, Su H. Wang L, et al. PLoS One. 2016 Apr 18;11(4):e0153835. doi: 10.1371/journal.pone.0153835. eCollection 2016. PLoS One. 2016. PMID: 27089041 Free PMC article.
References
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. - PubMed
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. - PubMed
- Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL52773/HL/NHLBI NIH HHS/United States
- R01 HL063894/HL/NHLBI NIH HHS/United States
- HL63894/HL/NHLBI NIH HHS/United States
- R01 NS032151/NS/NINDS NIH HHS/United States
- R01 HL052773/HL/NHLBI NIH HHS/United States
- R01NS32151/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases