O-GlcNAcylation: a novel pathway contributing to the effects of endothelin in the vasculature - PubMed (original) (raw)
Review
O-GlcNAcylation: a novel pathway contributing to the effects of endothelin in the vasculature
Victor V Lima et al. Am J Physiol Regul Integr Comp Physiol. 2011 Feb.
Abstract
Glycosylation with O-linked β-N-acetylglucosamine (O-GlcNAc) or O-GlcNAcylation on serine and threonine residues of nuclear and cytoplasmic proteins is a posttranslational modification that alters the function of numerous proteins important in vascular function, including kinases, phosphatases, transcription factors, and cytoskeletal proteins. O-GlcNAcylation is an innovative way to think about vascular signaling events both in physiological conditions and in disease states. This posttranslational modification interferes with vascular processes, mainly vascular reactivity, in conditions where endothelin-1 (ET-1) levels are augmented (e.g. salt-sensitive hypertension, ischemia/reperfusion, and stroke). ET-1 plays a crucial role in the vascular function of most organ systems, both in physiological and pathophysiological conditions. Recognition of ET-1 by the ET(A) and ET(B) receptors activates intracellular signaling pathways and cascades that result in rapid and long-term alterations in vascular activity and function. Components of these ET-1-activated signaling pathways (e.g., mitogen-activated protein kinases, protein kinase C, RhoA/Rho kinase) are also targets for O-GlcNAcylation. Recent experimental evidence suggests that ET-1 directly activates O-GlcNAcylation, and this posttranslational modification mediates important vascular effects of the peptide. This review focuses on ET-1-activated signaling pathways that can be modified by O-GlcNAcylation. A brief description of the O-GlcNAcylation biology is presented, and its role on vascular function is addressed. ET-1-induced O-GlcNAcylation and its implications for vascular function are then discussed. Finally, the interplay between O-GlcNAcylation and O-phosphorylation is addressed.
Figures
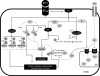
Fig. 1.
Signaling pathways activated by endothelin-1 (ET-1) in vascular smooth muscle cells (VSMC). Recognition of ET-1 by the ETA and ETB receptors in VSMCs activates intracellular signaling pathways and cascades that result in rapid alterations in cell activity and function and initiates transcriptional responses. Highlighted in the figure is the activation of PKC, MAPKs, and RhoA/Rho kinase signaling pathways, with subsequent effects on intracellular Ca2+ and calmodulin-dependent pathways as well as Ca2+-independent pathways. IP3, inositol 1,4,5-trisphosphate; SRC, sarcoma; P, phosphorylation; Pyk2, proline-rich tyrosine kinase-2; GDI, GDP dissociation inhibitors; MEK, MAPK kinase; MLC, myosin light chain; IP3R, IP3, repector; SR, sarcoplasmic reticulum.
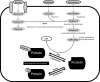
Fig. 2.
Hexosamine biosynthetic pathway (HBP). Enzymes involved in the synthesis of uridine-diphosphate-_O_-GlcNAcylation (UDP-_O_-GlcNAc) from glucose and glucosamine and the _O_-GlcNAc modification of proteins are shown. Depicted in the figure is the interplay between _O_-GlcNAc and _O_-phosphorylation.
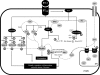
Fig. 3.
Proteins of signaling pathways activated by ET-1 are targets for _O_-GlcNAc. Indicated in the figure are signaling proteins that play an important role in the vascular effects by ET-1 and that are subject to the posttranslational modification by _O_-GlcNAc (please refer to the text for more details and Fig. 1 for abbreviations). G, targets of GlcNAcylation.
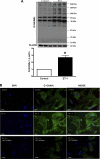
Fig. 4.
A: ET-1 increases the content of _O_-GlcNAc-proteins in rat aorta after 24-h incubation. Top: representative Western blot image of _O_-GlcNAc-proteins. Bottom: corresponding bar graphs showing the relative _O_-GlcNAc-proteins after normalization to β-actin expression. Results are presented as means ± SE for n = 6 in each experimental group. *P < 0.05 vs. control. B: ET-1 effects on _O_-GlcNAc protein levels are not observed when vessels were previously incubated with a selective β-_N_-acetylglucosaminidase (OGT) inhibitor. Phase contrast microscopy demonstrating that ET-1 increases _O_-GlcNAc-proteins in vascular smooth muscle cells (in culture). Previous incubation (3 h) of cells with an OGT inhibitor (3-[2-adamantanylethyl]-2-[4-chlorophenylazamethylene]-4-oxo-1,3-thiazaperhyd roine-6-carboxylic acid; 100 μmol/l) abrogates ET-1 effects on _O_-GlcNAc levels. Blue, DAPI stained nuclei; green, _O_-GlcNAc-modified proteins [FITC-labeled second antibody (anti-mouse IgG) and primary anti-_O_-GlcNAc antibody]. _O_-GlcNAc levels, before (top) and after PugNAc stimulation (bottom). Magnification, ×20.
Similar articles
- O-GlcNAcylation contributes to the vascular effects of ET-1 via activation of the RhoA/Rho-kinase pathway.
Lima VV, Giachini FR, Carneiro FS, Carvalho MH, Fortes ZB, Webb RC, Tostes RC. Lima VV, et al. Cardiovasc Res. 2011 Feb 15;89(3):614-22. doi: 10.1093/cvr/cvq338. Epub 2010 Oct 26. Cardiovasc Res. 2011. PMID: 20978008 Free PMC article. - Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system.
Laczy B, Hill BG, Wang K, Paterson AJ, White CR, Xing D, Chen YF, Darley-Usmar V, Oparil S, Chatham JC. Laczy B, et al. Am J Physiol Heart Circ Physiol. 2009 Jan;296(1):H13-28. doi: 10.1152/ajpheart.01056.2008. Epub 2008 Nov 21. Am J Physiol Heart Circ Physiol. 2009. PMID: 19028792 Free PMC article. - O-GlcNAcylation contributes to augmented vascular reactivity induced by endothelin 1.
Lima VV, Giachini FR, Carneiro FS, Carneiro ZN, Saleh MA, Pollock DM, Fortes ZB, Carvalho MH, Ergul A, Webb RC, Tostes RC. Lima VV, et al. Hypertension. 2010 Jan;55(1):180-8. doi: 10.1161/HYPERTENSIONAHA.109.143818. Epub 2009 Nov 30. Hypertension. 2010. PMID: 19948983 Free PMC article. - O-GlcNAcylation and oxidation of proteins: is signalling in the cardiovascular system becoming sweeter?
Lima VV, Spitler K, Choi H, Webb RC, Tostes RC. Lima VV, et al. Clin Sci (Lond). 2012 Oct;123(8):473-86. doi: 10.1042/CS20110638. Clin Sci (Lond). 2012. PMID: 22757958 Free PMC article. Review. - The hexosamine signaling pathway: deciphering the "O-GlcNAc code".
Love DC, Hanover JA. Love DC, et al. Sci STKE. 2005 Nov 29;2005(312):re13. doi: 10.1126/stke.3122005re13. Sci STKE. 2005. PMID: 16317114 Review.
Cited by
- Protein O-linked β-N-acetylglucosamine: a novel effector of cardiomyocyte metabolism and function.
Darley-Usmar VM, Ball LE, Chatham JC. Darley-Usmar VM, et al. J Mol Cell Cardiol. 2012 Mar;52(3):538-49. doi: 10.1016/j.yjmcc.2011.08.009. Epub 2011 Aug 22. J Mol Cell Cardiol. 2012. PMID: 21878340 Free PMC article. Review. - Role of Posttranslational Modifications of Proteins in Cardiovascular Disease.
Liu YP, Zhang TN, Wen R, Liu CF, Yang N. Liu YP, et al. Oxid Med Cell Longev. 2022 Jul 9;2022:3137329. doi: 10.1155/2022/3137329. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35855865 Free PMC article. Review. - The roles of O-linked β-N-acetylglucosamine in cardiovascular physiology and disease.
Zachara NE. Zachara NE. Am J Physiol Heart Circ Physiol. 2012 May 15;302(10):H1905-18. doi: 10.1152/ajpheart.00445.2011. Epub 2012 Jan 27. Am J Physiol Heart Circ Physiol. 2012. PMID: 22287582 Free PMC article. Review. - Cardiovascular disease in diabetes: where does glucose fit in?
Reusch JE, Wang CC. Reusch JE, et al. J Clin Endocrinol Metab. 2011 Aug;96(8):2367-76. doi: 10.1210/jc.2010-3011. Epub 2011 May 18. J Clin Endocrinol Metab. 2011. PMID: 21593112 Free PMC article. Review.
References
- Allahdadi KJ, Walker BR, Kanagy NL. ROK contribution to endothelin-mediated contraction in aorta and mesenteric arteries following intermittent hypoxia/hypercapnia in rats. Am J Physiol Heart Circ Physiol 293: H2911–H2918, 2007 - PubMed
- Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature 348: 730–732, 1990 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources