Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy - PubMed (original) (raw)
Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy
Xi-Nian Zuo et al. J Neurosci. 2010.
Abstract
Functional homotopy, the high degree of synchrony in spontaneous activity between geometrically corresponding interhemispheric (i.e., homotopic) regions, is a fundamental characteristic of the intrinsic functional architecture of the brain. However, despite its prominence, the lifespan development of the homotopic resting-state functional connectivity (RSFC) of the human brain is rarely directly examined in functional magnetic resonance imaging studies. Here, we systematically investigated age-related changes in homotopic RSFC in 214 healthy individuals ranging in age from 7 to 85 years. We observed marked age-related changes in homotopic RSFC with regionally specific developmental trajectories of varying levels of complexity. Sensorimotor regions tended to show increasing homotopic RSFC, whereas higher-order processing regions showed decreasing connectivity (i.e., increasing segregation) with age. More complex maturational curves were also detected, with regions such as the insula and lingual gyrus exhibiting quadratic trajectories and the superior frontal gyrus and putamen exhibiting cubic trajectories. Sex-related differences in the developmental trajectory of functional homotopy were detected within dorsolateral prefrontal cortex (Brodmann areas 9 and 46) and amygdala. Evidence of robust developmental effects in homotopic RSFC across the lifespan should serve to motivate studies of the physiological mechanisms underlying functional homotopy in neurodegenerative and psychiatric disorders.
Figures
Figure 1.
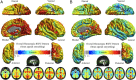
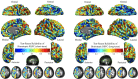
Whole-brain homotopic RSFC pattern. A multiple linear regression models the mean of VMHC at each voxel. One-sample tests on the individual VMHC values were performed. Correction for multiple comparisons was performed within one hemisphere (only one correlation for each pair of homotopic voxels) based on Gaussian random field theory (minimum Z > 2.3; cluster level, p < 0.05, corrected). The final statistical maps are visualized as six hemispheric surfaces (cortical regions) and six symmetric axial slices (subcortical regions) for both 6 mm FWHM (A) and 0 mm FWHM (B) spatial smoothing preprocessing strategies.
Figure 2.
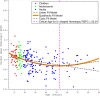
Scatter plots of global homotopic RSFC. The global homotopic RSFC was obtained for each subject by averaging VMHC values across all gray matter voxels within a predefined gray matter mask (40% threshold) in MNI152 standard space. Linear, quadratic, and cubic curve fittings were applied to these data. Three fit curves are plotted. The quadratic fitting was chosen as the best-fit model using AIC-based model selection, and its peak age is also plotted.
Figure 3.
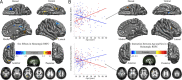
Developmental trajectories of voxelwise homotopic RSFC. Multiple linear regressions model the linear (Lin), quadratic (Qua), and cubic (Cub) age effects on VMHC at each voxel. AIC selected the best model from these three models. One-sample tests on the regression coefficients of age, age2, and age3 variables were performed for both positive (+) and negative (−) contrasts. Multiple comparisons correction was performed within one hemisphere (only one correlation for each pair of homotopic voxels) based on Gaussian random field theory (minimum Z > 2.3; cluster level, p < 0.05, corrected). All six statistical _Z_-maps (3 models × 2 contrasts) were binarized and combined into one summary map to depict different development trajectories of VMHC. The final maps were visualized as six hemispheric surfaces (cortical regions) and six hemispheric axial slices (subcortical regions). The right plots showed the developmental trajectory of a significant cluster for each of six types of trajectories: middle frontal gyrus (MFG: Lin+), anterior cingulate cortex (ACC: Lin−), insula (INS: Qua+), lingual gyrus (LG: Qua−), superior frontal gyrus (SFG: Cub+), and putamen (PUT: Cub−).
Figure 4.
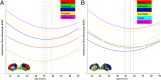
Trajectories of homotopic RSFC in large brain lobar/hierarchical subdivisions. Quadratic regression analyses selected by AIC on age and the mean VMHC of five brain structural subdivisions (A: frontal, temporal, parietal, and occipital lobes and subcortical region) or six hierarchical subdivisions (B: primary, unimodal, heteromodal, paralimbic, limbic, and subcortical) were conducted. These large brain units are visualized on the standard brain medial and lateral surfaces. The fit curves and their peak ages are plotted with the same color indicated on the surfaces. All the curve fits are shown significant after Bonferroni's correction.
Figure 5.
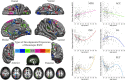
Sex effects of homotopic RSFC. Multiple linear regressions model the age, sex, and their interaction effects on VMHC at each voxel. One-sample tests on regression coefficients of both sex (A) and age × sex interaction (B) were performed for both positive and negative contrasts. Multiple comparisons correction was performed within one hemisphere (only one correlation for each pair of homotopic voxels) based on Gaussian random field theory (minimum Z > 2.3; cluster level, p < 0.05, corrected). The final statistical maps are visualized as six hemispheric surfaces (cortical regions) and six hemispheric axial slices (subcortical regions). The two scatter plots showed the details of the interactions between age and sex (male, red; female, blue).
Figure 6.
Test–retest reliability of homotopic RSFC. This figure depicts the voxelwise intraclass correlation (ICC) maps showing the intrasession or short-term (A) and intersession or long-term (B) test–retest reliability for homotopic RSFC. The final intraclass correlation maps are visualized as six hemispheric surfaces (cortical regions) and six symmetric axial slices (subcortical regions).
Similar articles
- Developmental sex differences in resting state functional connectivity of amygdala sub-regions.
Alarcón G, Cservenka A, Rudolph MD, Fair DA, Nagel BJ. Alarcón G, et al. Neuroimage. 2015 Jul 15;115:235-44. doi: 10.1016/j.neuroimage.2015.04.013. Epub 2015 Apr 14. Neuroimage. 2015. PMID: 25887261 Free PMC article. - Decreased homotopic interhemispheric functional connectivity in children with autism spectrum disorder.
Yao S, Zhou M, Zhang Y, Zhou F, Zhang Q, Zhao Z, Jiang X, Xu X, Becker B, Kendrick KM. Yao S, et al. Autism Res. 2021 Aug;14(8):1609-1620. doi: 10.1002/aur.2523. Epub 2021 Apr 28. Autism Res. 2021. PMID: 33908177 - Intelligence-related differences in the asymmetry of spontaneous cerebral activity.
Santarnecchi E, Tatti E, Rossi S, Serino V, Rossi A. Santarnecchi E, et al. Hum Brain Mapp. 2015 Sep;36(9):3586-602. doi: 10.1002/hbm.22864. Epub 2015 Jun 8. Hum Brain Mapp. 2015. PMID: 26059228 Free PMC article. - A surface-based technique for mapping homotopic interhemispheric connectivity: Development, characterization, and clinical application.
Tobyne SM, Boratyn D, Johnson JA, Greve DN, Mainero C, Klawiter EC. Tobyne SM, et al. Hum Brain Mapp. 2016 Aug;37(8):2849-68. doi: 10.1002/hbm.23214. Epub 2016 May 24. Hum Brain Mapp. 2016. PMID: 27219660 Free PMC article. - AICHA: An atlas of intrinsic connectivity of homotopic areas.
Joliot M, Jobard G, Naveau M, Delcroix N, Petit L, Zago L, Crivello F, Mellet E, Mazoyer B, Tzourio-Mazoyer N. Joliot M, et al. J Neurosci Methods. 2015 Oct 30;254:46-59. doi: 10.1016/j.jneumeth.2015.07.013. Epub 2015 Jul 23. J Neurosci Methods. 2015. PMID: 26213217
Cited by
- Brain Morphological and Functional Changes in Adenomyosis with Pain: A Resting State Functional Magnetic Resonance Imaging Study.
Jiao X, Yuan M, Li Q, Huang Y, Ji M, Li J, Yan S, Sun H, Wang X, Pan Z, Ren Q, Wang D, Wang G. Jiao X, et al. J Clin Med. 2022 Sep 7;11(18):5286. doi: 10.3390/jcm11185286. J Clin Med. 2022. PMID: 36142933 Free PMC article. - Sex differences in brain homotopic co-activations: a meta-analytic study.
Bonelli C, Mancuso L, Manuello J, Liloia D, Costa T, Cauda F. Bonelli C, et al. Brain Struct Funct. 2022 Nov;227(8):2839-2855. doi: 10.1007/s00429-022-02572-0. Epub 2022 Oct 21. Brain Struct Funct. 2022. PMID: 36269398 Free PMC article. - Ventral medial prefrontal functional connectivity and emotion regulation in chronic schizophrenia: a pilot study.
Fan FM, Tan SP, Yang FD, Tan YL, Zhao YL, Chen N, Li BB, Song CS, Wang YH, Jin Z, Zhou DF, Milham MP, Zou YZ, Zuo XN. Fan FM, et al. Neurosci Bull. 2013 Feb;29(1):59-74. doi: 10.1007/s12264-013-1300-8. Epub 2013 Jan 14. Neurosci Bull. 2013. PMID: 23319314 Free PMC article. - Optimally weighted L(2) distance for functional data.
Chen H, Reiss PT, Tarpey T. Chen H, et al. Biometrics. 2014 Sep;70(3):516-25. doi: 10.1111/biom.12161. Epub 2014 Mar 13. Biometrics. 2014. PMID: 26228660 Free PMC article. - The influence of mild carbon dioxide on brain functional homotopy using resting-state fMRI.
Marshall O, Uh J, Lurie D, Lu H, Milham MP, Ge Y. Marshall O, et al. Hum Brain Mapp. 2015 Oct;36(10):3912-21. doi: 10.1002/hbm.22886. Epub 2015 Jul 2. Hum Brain Mapp. 2015. PMID: 26138728 Free PMC article.
References
- Akaike H. A new look at statistical-model identification. IEEE Trans Automat Contr. 1974;19:716–723.
- Andersen R. Nonparametric methods for modeling nonlinearity in regression analysis. Annu Rev Sociol. 2009;35:67–85.
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical