TRPA1 contributes to cold hypersensitivity - PubMed (original) (raw)
. 2010 Nov 10;30(45):15165-74.
doi: 10.1523/JNEUROSCI.2580-10.2010.
Sarah Murphy, Melissa Heiry, Lee B Barrett, Taryn J Earley, Colby A Cook, Matt J Petrus, Michael Zhao, Marc D'Amours, Nate Deering, Gary J Brenner, Michael Costigan, Neil J Hayward, Jayhong A Chong, Christopher M Fanger, Clifford J Woolf, Ardem Patapoutian, Magdalene M Moran
Affiliations
- PMID: 21068322
- PMCID: PMC3021322
- DOI: 10.1523/JNEUROSCI.2580-10.2010
TRPA1 contributes to cold hypersensitivity
Donato del Camino et al. J Neurosci. 2010.
Abstract
TRPA1 is a nonselective cation channel expressed by nociceptors. Although it is widely accepted that TRPA1 serves as a broad irritancy receptor for a variety of reactive chemicals, its role in cold sensation remains controversial. Here, we demonstrate that mild cooling markedly increases agonist-evoked rat TRPA1 currents. In the absence of an agonist, even noxious cold only increases current amplitude slightly. These results suggest that TRPA1 is a key mediator of cold hypersensitivity in pathological conditions in which reactive oxygen species and proinflammatory activators of the channel are present, but likely plays a comparatively minor role in acute cold sensation. Supporting this, cold hypersensitivity can be induced in wild-type but not Trpa1(-/-) mice by subcutaneous administration of a TRPA1 agonist. Furthermore, the selective TRPA1 antagonist HC-030031 [2-(1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydro-7H-purin-7-yl)-N-(4-isopropylphenyl)acetamide] reduces cold hypersensitivity in rodent models of inflammatory and neuropathic pain.
Figures
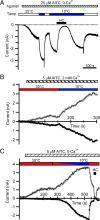
Figure 1.
Cooling activates TRPA1 currents. A, Whole-cell recording of an HEK-293 cell expressing rat TRPA1 (rTRPA1). A 400 ms ramp from −100 to 100 mV was applied every 5 s, and current measured at −80 mV (filled circles) and +80 mV (open circles) during each ramp is plotted as a function of time. The white and blue horizontal bars indicate the perfusion of bath solution at 25 or 10°C, respectively. The 20 μ
m
HC-030031 at 10°C was added during the time indicated by the black horizontal bar. B, Current–voltage relationships recorded from the cell shown in A collected at the indicated times (1, 2, and 3). C, Mean inward (at −80 mV, black bars, downward) and outward (at +80 mV, gray bars, upward) current from four cells at baseline (25°C), after cooling (10°C), and after treatment with 25 μ
m
AITC at 25°C. The 20 μ
m
HC-030031 was used to determine TRPA1-specific component of the currents. Data are plotted as mean ± SEM. D, Fold increase in inward (at −80 mV, black bars, downward) and outward (at 80 mV, gray bars, upward) current over baseline at 25°C with cooling (10°C), and after treatment with 25 μ
m
AITC at 25°C. Data from the same cells as in C are plotted as mean ± SEM.
Figure 2.
Rapid cooling potentiates TRPA1 currents after activation by agonist. A, Continuous whole-cell recordings at −60 mV from a representative HEK-293 cell expressing rTRPA1. Ca2+-free bath solutions were perfused at either 25°C (open horizontal bars) or at 10°C (blue horizontal bars). AITC was added during the time indicated by the horizontal bar. The TRPA1-specific blocker HC-030031 was added at a concentration of 20 μ
m
(black bar; HC). The dotted line represents the 0 current level. Decreasing the temperature from 25 to 10°C potentiated the current 2.33-fold (±0.28; n = 4). B, Whole-cell recording from a representative HEK-293 cell expressing rTRPA1. From a holding potential of 0 mV, 400 ms ramps from −100 to 100 mV applied every 4 s were used to elicit inward (measured at −80 mV, filled circles) and outward (measured at 80 mV, open circles) currents, which are plotted as a function of time. The bath solution contained 2 m
m
Ca2+. As in A, a rapid temperature exchange was achieved using two different temperature control and perfusion systems preset at the indicated temperatures (30°C, red horizontal bars, or 10°C, blue horizontal bars) to ensure a rapid transition from 30 to 10°C within 15 s. C, Same as in B but without Ca2+ in the bath solution. Here, temperature is reduced from 30 to 10°C at a very slow rate (∼20°C/5 min). Under these conditions, lowering the temperature resulted in clear current potentiation.
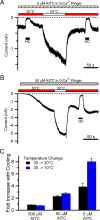
Figure 3.
Magnitude of cooling-induced TRPA1 potentiation is dependent on the temperature and AITC concentration. A, B, Representative continuous whole-cell recordings at −60 mV from HEK-293 cells expressing rTRPA1 and perfused with Ca2+-free bath solutions containing AITC at 5 μ
m
(A) or 50 μ
m
(B). AITC was perfused at 30°C (red horizontal bars) or 20°C (open horizontal bars). Addition of 5 μ
m
AITC caused a very slow activation of the current. For this reason, the early phase of current development is omitted in A. The 20 μ
m
HC-030031 was perfused at the times indicated by the black horizontal bars. C, Quantification of the increase in inward current evoked by cooling over that evoked by AITC alone. Cooling from 30 to 20°C (black bars) or from 30 to 10°C (blue bars) is shown in the presence of varying concentrations of AITC. The bars show mean ± SEM of three (500 μ
m
AITC) or four (50 and 5 μ
m
AITC) cells from whole-cell recordings as shown in A and B.
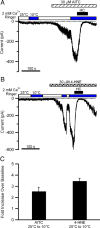
Figure 4.
Cooling potentiates AITC- or 4-HNE-activated currents in isolated DRG neurons. A, B, Continuous whole-cell recordings at −60 mV from isolated rat DRG neurons. The temperature of the perfusion solutions was switched from 25°C (open horizontal bars) to 10°C (blue horizontal bars) both in absence of an agonist and in the presence of AITC (A) or 4-HNE (B). The currents activated by those two agonists were blocked by 20 μ
m
HC-030031 (black horizontal bars). C, Quantification of the fold increase in inward current evoked by cooling from 25 to 10°C in the presence of AITC (30 μ
m
) or 4-HNE (30 μ
m
). The bars represent the mean ± SEM of four (AITC) or five (4-HNE) DRG neurons.
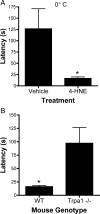
Figure 5.
Five millimolar 4-HNE induces TRPA1-dependent cold allodynia. A, B, Mice were injected with 5 m
m
4-HNE or vehicle and then placed on a 0°C cold plate and observed for latency to first pain response. A, Cold responses from vehicle-treated and 5 m
m
4-HNE-treated mice are compared. B, Cold responses from 5 m
m
4-HNE-treated WT and Trpa1 −/− mice are compared. The bars represent the mean ± SEM (**p < 0.01).
Figure 6.
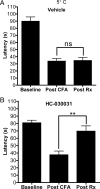
HC-030031 ameliorates CFA-induced cold allodynia. A, B, The latency to first pain response on a 5°C cold plate was measured in rats before injection of CFA into one hindpaw (baseline), 48 h after injection of CFA (post-CFA), and again after treatment with either HC-030031 or vehicle (post-Rx). Paws injected with CFA are evaluated at baseline, post-CFA, and post-RX with vehicle (A) and HC-030031 (B). The bars represent the mean ± SEM (n = 7) (**p < 0.01).
Figure 7.
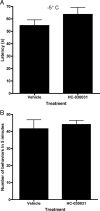
HC-030031 has no effect on the detection of noxious cold in the absence of CFA injection. Rats injected with either HC-030031 or vehicle were placed on a −5°C cold plate. A, Latency to first response was measured in vehicle- or HC-030031-treated animals. The bars represent the mean ± SEM (n = 16). B, Number of pain behaviors exhibited in 5 min was measured in vehicle- or HC-030031-treated animals. The bars represent the mean ± SEM (n = 8).
Figure 8.
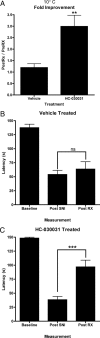
Nerve injury in rats induces cold allodynia that is ameliorated by blocking TRPA1. A, The latency to first reaction to placement on a 10°C cold plate was determined in rats 7 d after SNI surgery (n = 10). The response latency was then measured after treatment with either vehicle or HC-030031 and is presented as a ratio of posttreatment to pretreatment response latency (**p < 0.01). B, C, Response latency of rats after placement on a 10°C cold plate before SNI, 7 d after SNI, and 1 h after treatment with vehicle (B) or HC-030031 (C) was measured (***p < 0.001). The bars represent the mean ± SEM.
Similar articles
- The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity.
Gentry C, Stoakley N, Andersson DA, Bevan S. Gentry C, et al. Mol Pain. 2010 Jan 21;6:4. doi: 10.1186/1744-8069-6-4. Mol Pain. 2010. PMID: 20092626 Free PMC article. - TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons: pharmacological and genetic evidence.
Fajardo O, Meseguer V, Belmonte C, Viana F. Fajardo O, et al. J Neurosci. 2008 Jul 30;28(31):7863-75. doi: 10.1523/JNEUROSCI.1696-08.2008. J Neurosci. 2008. PMID: 18667618 Free PMC article. - Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation.
Chen J, Joshi SK, DiDomenico S, Perner RJ, Mikusa JP, Gauvin DM, Segreti JA, Han P, Zhang XF, Niforatos W, Bianchi BR, Baker SJ, Zhong C, Simler GH, McDonald HA, Schmidt RG, McGaraughty SP, Chu KL, Faltynek CR, Kort ME, Reilly RM, Kym PR. Chen J, et al. Pain. 2011 May;152(5):1165-1172. doi: 10.1016/j.pain.2011.01.049. Epub 2011 Mar 12. Pain. 2011. PMID: 21402443 - How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation.
McKemy DD. McKemy DD. Mol Pain. 2005 Apr 22;1:16. doi: 10.1186/1744-8069-1-16. Mol Pain. 2005. PMID: 15847696 Free PMC article. Review. - The molecular and cellular basis of cold sensation.
McKemy DD. McKemy DD. ACS Chem Neurosci. 2013 Feb 20;4(2):238-47. doi: 10.1021/cn300193h. Epub 2012 Nov 28. ACS Chem Neurosci. 2013. PMID: 23421674 Free PMC article. Review.
Cited by
- Scratching the surface: a role of pain-sensing TRPA1 in itch.
Xiao B, Patapoutian A. Xiao B, et al. Nat Neurosci. 2011 May;14(5):540-2. doi: 10.1038/nn.2813. Nat Neurosci. 2011. PMID: 21522144 No abstract available. - Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics.
Sousa-Valente J, Andreou AP, Urban L, Nagy I. Sousa-Valente J, et al. Br J Pharmacol. 2014 May;171(10):2508-27. doi: 10.1111/bph.12532. Br J Pharmacol. 2014. PMID: 24283624 Free PMC article. Review. - Molecular mechanisms of temperature adaptation.
Bagriantsev SN, Gracheva EO. Bagriantsev SN, et al. J Physiol. 2015 Aug 15;593(16):3483-91. doi: 10.1113/jphysiol.2014.280446. Epub 2015 Jan 5. J Physiol. 2015. PMID: 25433072 Free PMC article. Review. - Transient receptor potential ankyrin 1 that is induced in dorsal root ganglion neurons contributes to acute cold hypersensitivity after oxaliplatin administration.
Yamamoto K, Chiba N, Chiba T, Kambe T, Abe K, Kawakami K, Utsunomiya I, Taguchi K. Yamamoto K, et al. Mol Pain. 2015 Nov 13;11:69. doi: 10.1186/s12990-015-0072-8. Mol Pain. 2015. PMID: 26567040 Free PMC article. - 5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals.
Sisignano M, Park CK, Angioni C, Zhang DD, von Hehn C, Cobos EJ, Ghasemlou N, Xu ZZ, Kumaran V, Lu R, Grant A, Fischer MJ, Schmidtko A, Reeh P, Ji RR, Woolf CJ, Geisslinger G, Scholich K, Brenneis C. Sisignano M, et al. J Neurosci. 2012 May 2;32(18):6364-72. doi: 10.1523/JNEUROSCI.5793-11.2012. J Neurosci. 2012. PMID: 22553041 Free PMC article.
References
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. - PubMed
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS039518/NS/NINDS NIH HHS/United States
- R01 NS039518-09/NS/NINDS NIH HHS/United States
- R37 NS039518/NS/NINDS NIH HHS/United States
- NS039518/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases